How many moles are in 98.3 grams of aluminum hydroxide?
1.26 moles
What is the periodic trend for electron affinity?
As you go up a group and across a period from left to right, electron affinity increases (there are some exceptions; it has to do with stability of filled and half-filled shells).
What is the difference between an isotope and an ion?
Ions have different numbers of electrons, isotopes have different numbers of neutrons.
Why does the 4s orbital listed before the 3d orbitala in electron configurations?
The 4s orbital is lower in energy than the 3d orbital.
How many electrons can fit within the n=2 shell?
Hint: Each subshell can hold 2 electrons
10 electrons
n=1 --> 2 electrons total (s orbtial)
n=2 --> (s orbital and p orbital) 8 total electrons
10 overall
The experiment that led to the discovery of the atom's nucleus
Rutherford Gold Foil Experiment
Describe the periodic trend for increasing atomic radius. What is the largest element on the periodic table?
As you go DOWN a group and across a period from RIGHT TO LEFT, atomic radius increases. Therefore, francium is the largest element on the periodic table (bottom left-hand corner).
A cation is positively-charged. Has it lost or gained electrons? Which elements are most likely to become cations?
Metals
Write the electron configuration for iodine, NO ABBREVIATIONS!
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5
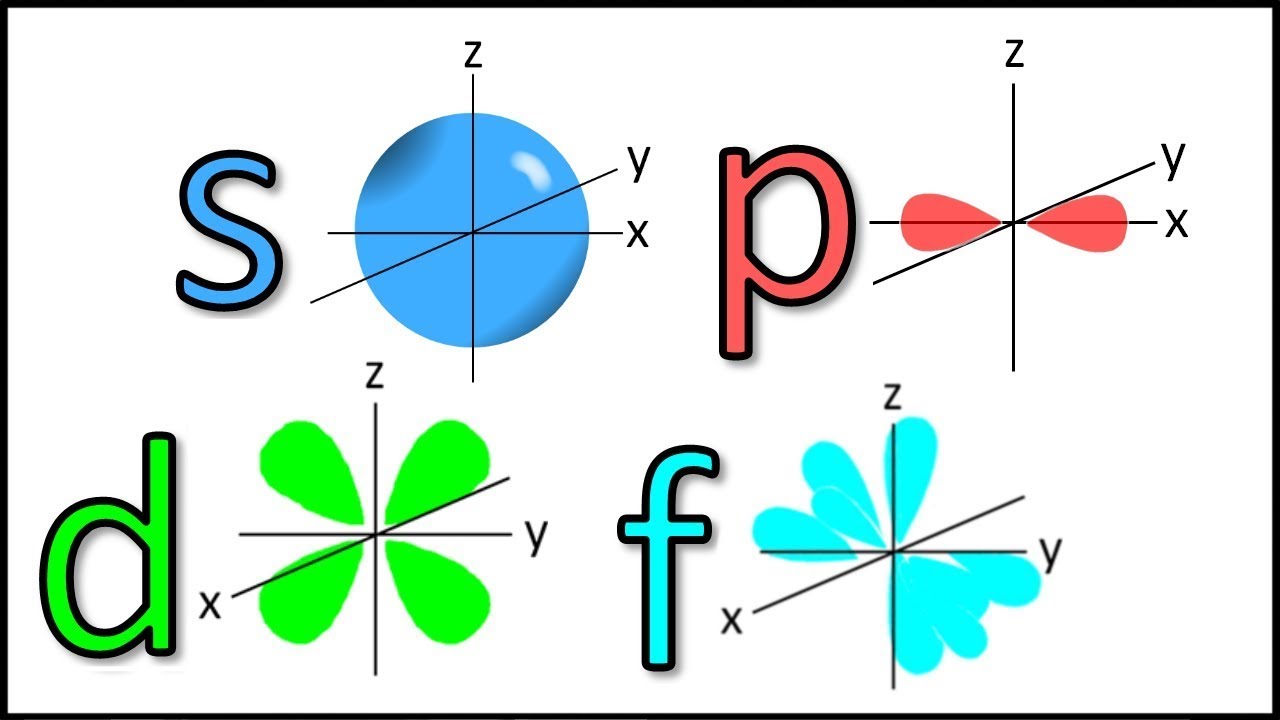
Draw the general (not specific orientations) shapes of an s orbital, a p orbital, and a d orbital.
The experiment that determined that the charge of a single electron was 1.6 x 10-19 C.
Millikan Oil Drop Experiment
Describe the trend in electronegativity.
What is the most electronegative element?
As you go UP a group and across a period from LEFT TO RIGHT, electronegativity INCREASES. (F is most electronegative)
3. An element has three isotopes. Given the abundances and relative masses, calculate the average atomic mass and determine (from the periodic table) which element it is.
Abundances Relative masses
0.005% 234.040947 amu
0.720% 235.043924 amu
99.275% 238.050784 amu
URANIUM
Avg atomic mass = 238.03 amu
USE ABBREVIATION
[Ne] 3s2 3p5
A hypothetical wave has 6.6 J of energy. What is its hypothetical, approximate frequency?
Hint: what is the relationship between energy and frequency involving Planck's constant (6.626 x 10^-34 J x s)?
0.9×10^34 Hz
The experiment that led to the discovery of the electron
J.J Thomson's Cathode Ray Tube Experiment
Dsecribe the trend in ionization energy.
What accounts for major "jumps" in ionization energies?
As you go up a group and across a period from left to right, ionization energy increases.
Major jumps in IE are due to removal of core electrons (WAY HARDER = MORE ENERGY REQUIRED).
What is the charge on the Calcium ion that is isoelectronic with Argon?
+2
Which element does this configuration correspond to?
[Xe] 6s2 4f14 5d10 6p2
lead
In regards to the Electromagnetic Spectrum, what is the relationship between energy, frequency, and wavelength?
(How does each change relative to the other)?
Energy & frequency are DIRECTLY proportional, while wavelength is INVERSELY proportional to both
What is unique about light as a wave?
The speed of light is CONSTANT (2.998 X 10^8 m/s), so we can figure out wavelength or frequency by manipulating the relationship:
c= (lambda)(nu)
What is the main determinant of ALL periodic trends?
Effective nuclear charge
16 protons
18 electrons
16 neutrons
What am I? (Write symbol and charge)
S ^ 2- (gained 2 electrons, anion)
Explain why chromium and copper embody EXCEPTIONS in their electron configurations.
STABILITY associated with half-filled and filled orbitals
"nature getting ahead of itself"
Describe the location/orientation of the electron with quantum numbers:
n=3
l = 0
ml = 0
ms= +1/2
3s orbital, spin up