You have 200.0 ml solution in which 145.0 g of KNO3 is dissolved. What would be the new volume if you diluted the solution to a molarity of 0.35 M?
a) 25.1 L
b) 2.51 L
c) 20.5 L
d) 2.05 L
a) 25.1 L
What does it mean to be Amphiprotic? Polyprotic? Give an example of each.
Amphiprotic - can be both acid and base (ex. H2O)
Polyprotic - an acid that can donate more than one proton or hydrogen atom per molecule to an aqueous solution. For polyprotic acids, the first Ka is always the largest, followed by the second, etc. This indicates that the protons become successively less acidic as they are lost. (ex. H2SO4, H3PO4)
Which of the following is an endothermic process?
a) jet fuel burning in a jet engine
b) combustion of methane
c) freezing of water
d) vaporization of water
d) vaporization of water
Ethanol (CH3CH2OH) has been suggested as an alternative fuel source. Ethanol’s enthalpy of combustion is ΔHcomb = −1368 kJ/mol, and its density is 0.789 g/mL. What is the fuel density of ethanol (kJ/mL)?
a) 3.75 kJ/mL
b) 12.8 kJ/mL
c) 1.28 kJ/mL
d) 23.4 kJ/mL
e) 37.5 kJ/mL
d) 23.4 kJ/mL
The surroundings perform work on a system while the system releases heat to the surroundings. Which of the following is true from the system’s perspective? (q = heat, w = work, ΔE = internal energy change)
a) q < 0, w > 0, ΔE > 0
b) q > 0, w < 0, ΔE > 0
c) q = −w, ΔE = 0
d) q > 0, w < 0, more information is needed to determine ΔE
e) q < 0, w > 0, more information is needed to determine ΔE
e) q < 0, w > 0, more information is needed to determine ΔE
In the reaction, 2 Al (s) + 6 HCl (aq) -> 2 AlCl3 (aq) + 3 H2 (g), 2.00 g of Al will react with how many milliliters of 0.500 M HCl?
a) 37.0 mL
b) 111 mL
c) 148 mL
d) 444 mL
e) None of the above
d) 444 mL
Select the net ionic equation for the reaction of sodium carbonate with hydrobromic acid.
a. Na2CO3 (aq) → 2Na+ (aq) +CO32− (aq)
b. HBrO3 (aq) → H+(aq) +BrO3− (aq)
c. Na+(aq) + Br− (aq) → NaBr (aq)
d. 2 Na+ (aq) +CO32− (aq) + 2 H+ (aq) + 2Br−(aq) → H2O(l) + 2Na(s) + CO2 (g) + Br2 (l)
e. 2H+ (aq) +CO32− (aq) → H2O(l) +CO2 (g)
e. 2H+ (aq) +CO32− (aq) → H2O(l) +CO2 (g)
Two aqueous solutions at room temp are mixed in a coffee cup calorimeter. The reaction causes the temp of the resulting solution to fall below room temp. Which of the following states is TRUE?
a) Energy is leaving the system during reaction
b) The products have a lower potential energy than the reactants
c) This type of experiment directly yields ΔErxn
d) The mixing is endothermic
e) The solution has special properties that enable it to violate the 1st & 2nd law of thermodynamics
d) The mixing is endothermic
Use the bond energies below to estimate the enthalpy change associated with the chlorination of methane to produce methylene chloride.
CH4 (g) + 2 Cl2 (g) → CH2Cl2 (g) + 2 HCl (g)
Cl—Cl: 243 kJ/mol; C—Cl: 328 kJ/mol
C—H: 413 kJ/mol; H—Cl: 431 kJ/mol
a) −103 kJ/mol
b) +103 kJ/mol
c) 376 kJ/mol
d) - 206 kJ/mol
e) 206 kJ/mol
d) - 206 kJ/mol
ΔHrxn = Σ[ΔH(bond breaking)] - Σ[ΔH(bond making)]
Bond breaking is endothermic. (Bonds in reactants are being broken.)
Bond making is exothermic. (Bonds are being made to produce products.)
A gas has a constant pressure of 50000Pa as it expands by 0.003m3. What is the net work performed on the gas?
a) 150J
b) 300J
c) 200J
d) 0J
e) 1500J
a) 150J
W= PΔV
W= (50000Pa) (0.003m3) = 150J.
You are given 100.0 mL of a 2.0 M solution of KOH. What would be the molarity of the solution if it is diluted to a volume of 1000 mL?
a) 2M
b) .02 M
c) .002 M
d) .2 M
d) 0.2 M
Balance the following redox reaction under acidic aqueous conditions using the smallest whole-number coefficients possible. What is the coefficient of H2O?
CrO42−(aq) + Cl2(aq) → OCl−(aq) + Cr3+(aq)
a) 2
b) 3
c) 4
d) 6
e) 12
a) 2
What does each letter stand for? And what type of reaction does this graph represent?
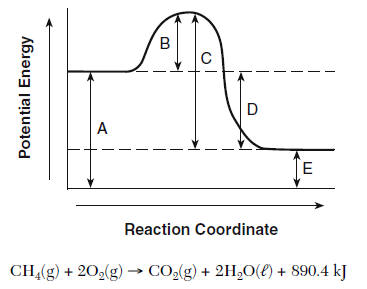
Exothermic
A: Potential Energy (PE) of reactants
B: Activation Energy (Ea)
C: Max Energy released from reaction
D: Net change in enthalpy (ΔH)
E: Potential Energy (PE) of products
Given the following reactions, what is the overall enthalpy change for the following reaction?
NH3 (g) +HCl(g) → NH4Cl(s)
Reaction ΔH° (kJ)
N2 (g) + 3 H2 (g) → 2 NH3 (g) −92
H2 (g) +Cl2 (g) → 2 HCl (g) −185
N2 (g) + 4 H2 (g) +Cl2 (g) → 2 NH4Cl (s) −629
a) −38 kJ
b) −176 kJ
c) −352 kJ
d) −445 kJ
e) −454 kJ
b) −176 kJ
1) A closed system contains 2g of ice. Another 2g of ice are added to the system. What is the final mass of the system?
2) An isolated system has an initial temperature of 30oC. It is then placed on top of a bunsen burner for an hour. What is the final temperature?
1) The final mass will be 2g. Remember, a closed system does not allow for mass exchange.
2) The final temperature will be 30oC. Remember, an isolated system does not allow energy transfer.
Lead levels in drinking water should be no higher than 15 ppb. What is this in mol/L? Assume the density of drinking water is 1.0 g/mL.
a) 7.2 × 10−8 M
b) 7.2 × 10−5 M
c) 3.1 × 10−7 M
d) 3.1 × 10−8 M
e) 2.0 × 10−8 M
a) 7.2 × 10−8 M
Balance the following redox reaction under basic aqueous conditions using the smallest whole-number coefficients possible. What is the coefficient of ClO3−?
ClO3−(aq) + S2O32−(aq) → Cl2(aq) + SO32-(aq)
a) 2
b) 3
c) 4
d) 5
e) 6
c) 4
A system absorbs heat Q and has an equal amount of positive work done on it. What is the change in the internal energy of the system?
a) Q2
b) −2Q (internal energy decreases)
c) 0
d) 2Q
e) Q
d) 2Q
Heat is absorbed so that is a +Q to internal energy. Also, positive work is done on the system so that is another +Q to internal energy. The total internal energy is increased by 2Q.
How much heat does it take to bring 0.5mol of ethanol from 60∘C to a saturated vapor? Use the following values for ethanol in your calculations:

a) 8.3 kJ
b) 33.3 kJ
c) 20.3 kJ
d) 26.9 kJ
e) 16.4 kJ
c) 20.3 kJ
Calculate how much energy is needed to bring the ethanol to its boiling point:
q=ncpΔT
q=(0.5mol)(112.4 J/K⋅mol)(78.37∘C−60∘C)
q=1032.4 J
Now we need to calculate the energy it takes to vaporize all of the ethanol:
q=nΔH∘vap
q=(0.5mol)(38.56 kJ/mol)
q=19.28 kJ
Now adding these together:
qtotal=20.3 kJ
In the cylinder of a certain internal combustion engine, the hot gas caused by the combustion of the fuel expands from V1=2.0∗10−4 m3 to a volume of V2=5.0∗10−4 m3 at a constant pressure of P=7.2∗107 Pa. How much work will the gas do on the piston during this expansion?
a) W= −3.60∗104 J
b) W=1.44∗104 J
c) W=2.16∗104 J
d) W=3.60∗104 J
e) W= −2.16∗104 J
c) W= 2.16∗104 J
W=PΔV=7.2∗107Pa ((5∗10−4m3)−(2∗10−4m3))
If I leave 750 mL of 0.50 M sodium chloride solution uncovered on a windowsill and 150 mL of the solvent evaporates, what will the new concentration of the sodium chloride solution be?
a) .033 M
b) .63 M
c) .86 M
d) .19 M
b) .63 M
It takes 12.5 mL of a 0.30 M HCl solution to neutralize 285 mL of NaOH solution. What is the concentration of the NaOH solution?
a) 0.36 M
b) 0.092 M
c) 0.16 M
d) 0.013 M
d) 0.013 M NaOH
HCl + NaOH -> NaCl + H2O
(MAVA)/nA = (MBVB)/nB
MB = (MAVA * nB)/(VBnA)
= (0.30 M)(12.5 mL)(1) / (285 mL)(1)
= 0.013 M NaOH
The graph represents the uniform heating of a substance, starting with the substance as a solid below its melting point. Which line segment listed below represents an increase in potential energy and no change in average kinetic energy?

B) BC
In an experiment, 30.0 g of metal is heated to 98.0°C and then quickly transferred to 50.0 g of water in a calorimeter at 27.0°C. The heat capacity of the calorimeter with the water is 211 J/°C. The final temperature comes to 32.5°C. What is the approximate specific heat capacity of the metal? [cp(water) = 4.18 J/(g ⋅ °C)]
a) 0.140 J/(g ⋅ °C)
b) 83.0 J/(g ⋅ °C)
c) 0.540 J/(g ⋅ °C)
d) 0.591 J/(g ⋅ °C)
e) 29.5 J/(g ⋅ °C)
d) 0.591 J/(g ⋅ °C)
A piston is filled with methane at a pressure of 3 atm with a current volume of 2L. If the methane doubles in volume isobarically (P stays constant), how much work does the gas do on the surrounding environment?
a) 2.8 kJ
b) 45 J
c) 608 J
d) 336 J
e) 1.3 kJ
C) 608 J
W=PΔV
W=(3atm)(2L)
W=(3atm)(2L)(101325 Pa/atm)(.001m3/L)
W=608J
