Which of the following substances has bonds with the greatest ionic character?
(A) KCl
(B) HCl
(C) CCl4
(D)NCl3
KCl the electronegativity difference is greatest.
3.2-0.8=2.4... no you do not need to memorize the electronegativities but you should be able to tell based on location on periodic table and the general trend. K is weakest so most ionic character.
What is an isomer?
compounds with same formula but different arrangement of atoms
The geometry of the CO2 molecule is best described as
(A) trigonal planar
(B) trigonal pyramidal
(C) square pyramidal
(D) bent
(E) linear
Linear
Which has the largest bond-dissociation energy?
(A) H2
(B) F2
(C) N2
(D) O2
N2
Would MgO have a higher melting point than that of NaF? Why?
Yes, because Mg2+ is more positively charged than Na+ and O2- is more negatively charged than F-
Intermolecular forces are stronger between Mg and O.
Draw BH3
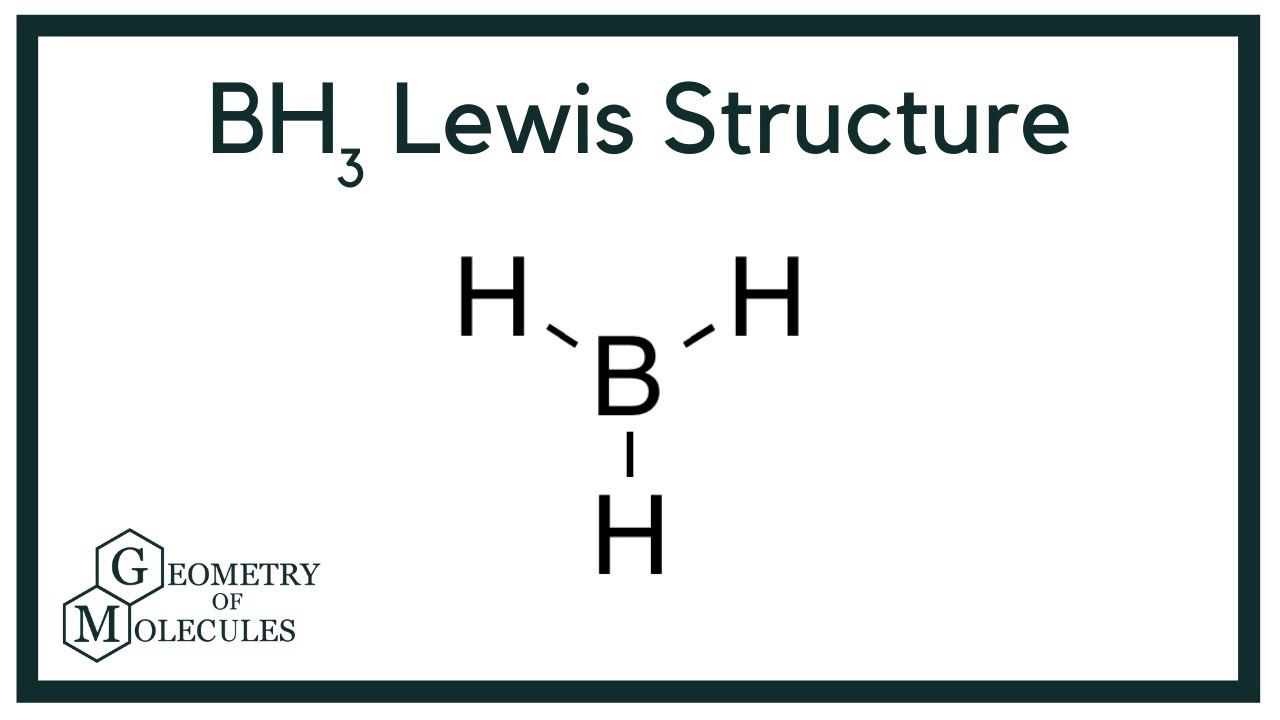
Do lone pairs count as a domain?
Yes
Of the following single bonds, which is the LEAST polar?
(A) H—F
(C) O—F
(D) I—F
(E) B—H
O--F
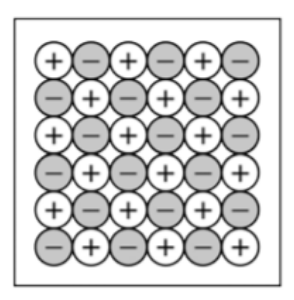
Draw solid KF particle diagram?
use a 6x6 block of atoms and include charges or letters for K and F
Name 2 elements break the octet rule?
B, S, P
Which has more electron domains?

SO2 has 3 and CO2 has 2
Which of the following molecules contains only single bonds?
(A) C2H6
(B) C6H6
(C) HCN
(D) CO2
C6H6
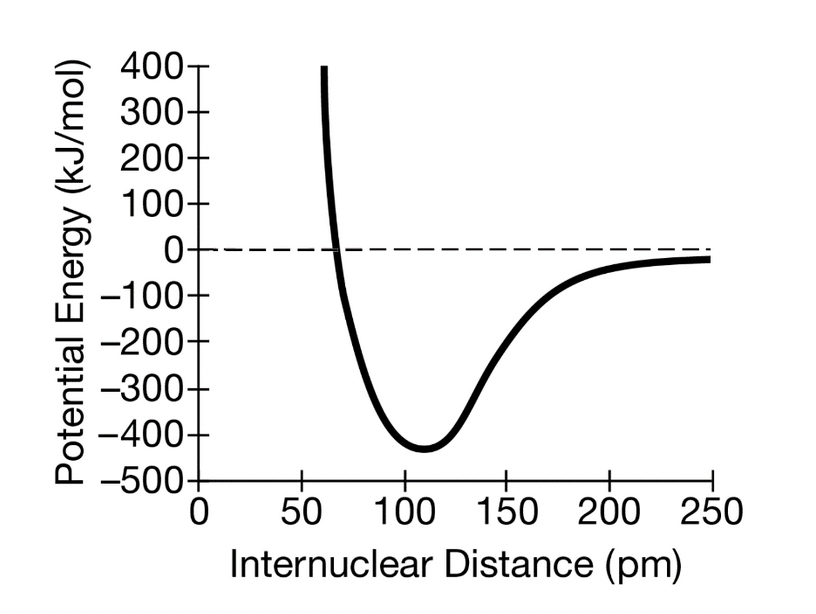
Draw what a potential energy (y-axis) versus internuclear distance (x-axis) graph might look like?
*numbers not necessary just the general curve
Which bond has two lone pairs
(A) H2O
(B) NH3
(C) BH3
(D) CH4
(E) SiH4
H2O
Which has a trigonal-pyramidal molecular geometry
(A) H2O
(B) NH3
(C) BH3
(D) CH4
(E) SiH4
NH3
Which of the following is an isomer of CH3OCH3 ?
(A) CH3CH3
(B) CH3COOH
(C) CH3CH2OH
(D) CH3CH2CH3
(E) CH3CH2OCH2CH3
CH3CH2OH
that was it.. so here's a more important question.
Arranges the molecules N2, O2, Cl2 in order of their bond enthalpies, from least to
greatest?
Cl2, O2, N2
think about it. Cl is a single bond, O2 is double and N2 is triple
Which of the following molecules contains polar covalent bonds but is a nonpolar molecule?
(A) CH3Cl
(B) CH2Cl2
(C) NH3
(D) CCl4
(E) H20
CCl4
if you put water, I am crying rn. H2O is THE most polar thing like ever. cmon. Let's talk I gotchu.
The hybridization of the carbon atom in the molecule represented can be described as what? with what bond angle?
CH4
sp3
109.5
Who has the greater dipole moment?
NCl3 or CS2
NH3
because the Cl draw electron density away from the N and the bond dipoles do
not cancel one another.