If the charge on the sigma complex is positive, would a Electron Donating or Withdrawing group stabilize it?
Electron DONATING group
KMnO4, Heat, -OH
Two concepts that describe stability in organic chemistry.
Inductive Effect
Resonance Effect
Why are carbonyl carbons electrophilic?
Location next to electronegative oxygen (see resonance structure)

An electron deficient species that is likely to accept lone pair electrons from another molecule
Electrophile

The stability of which reaction intermediate determines how "fast" a EAS reaction goes.
Sigma complex
Reaction conditions for a reduction
Zn(s), HCl
Sn(s), HCl
Fe(s), HCl
You can draw additional resonance structures using the lone pairs from the group
The purpose of OH- and H3O+ in Carbonyl Chemistry
OH- generates stronger nucleophiles
H3O+ generates a stronger electrophile (protonation of carbonyl)
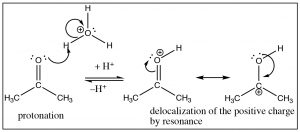
Electron rich species that can give electrons to electron poor species in a reaction mechanism.
Nucleophiles!

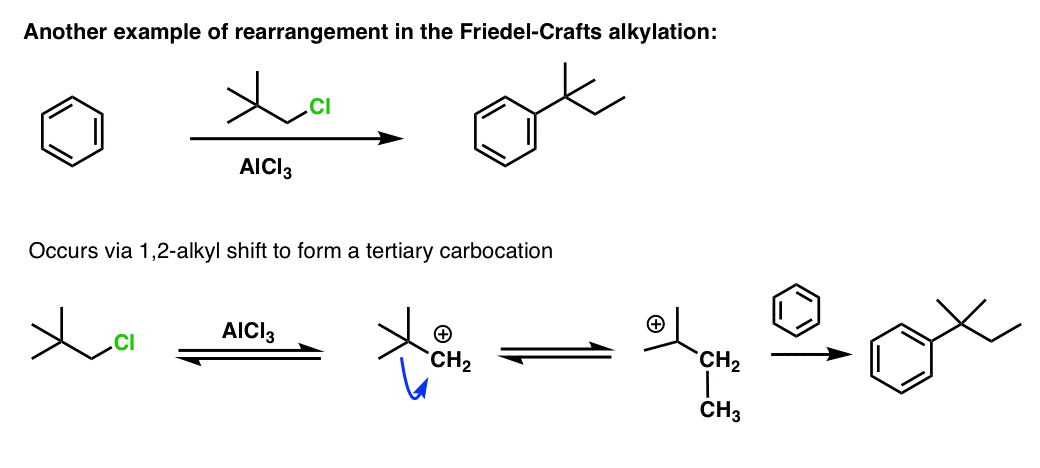
Friedel crafts alkylation are unreliable for what reasons?
Adds an electron donating group, so multiple additions occur.
Rearrangements of carbocation can occur.
In a synthesis, we try to perform a Friedel Crafts Acylation to the following molecule. Why is this a bad idea:
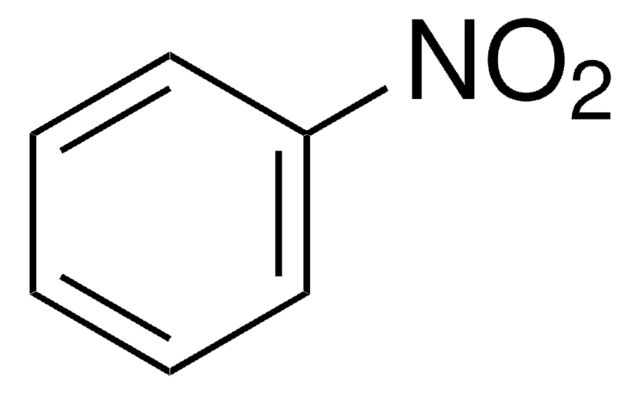
Ring is too strongly deactivated for a Friedel Crafts reaction.
Acetone is a liquid at room temperature. Describe the intermolecular forces acetone has, and why does it have a lower boiling point than water?
Acetone has only dipole dipole intermolecular forces, therefore is more volatile. Water is capable of H-bonding due to extremely polar O-H bond.
Electrophile --> Carbonyl carbon and carbon double bonded to imine.
Nucleophile --> Any electronegative atom (nitrogen, oxygen).
Under Acidic conditions, what chemical species should NOT be drawn in the mechanism.
Any base, or anything with a negative charge on it.
Draw two electrophiles that are involved in EAS reaction mechanisms.
(Note: There are more)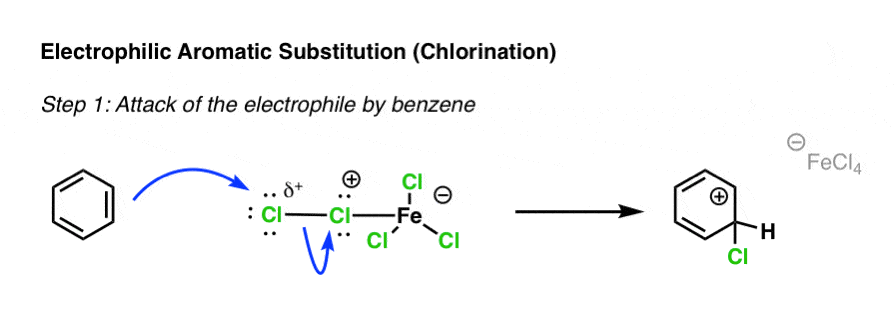
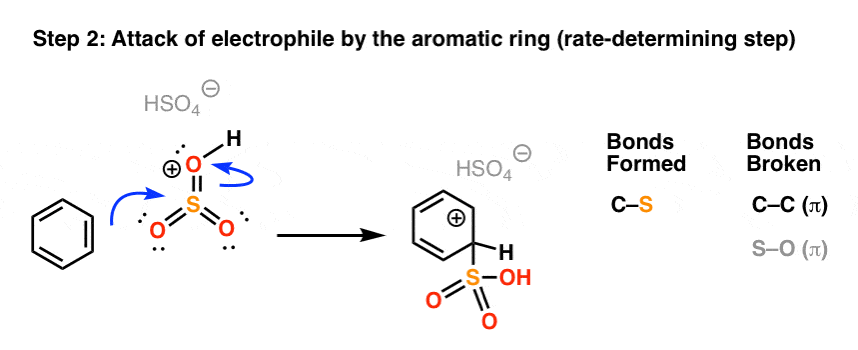
Why can't we make the following molecule from toluene in one step:

If two "directing" groups are on an aromatic ring, who wins:
Stronger Electron Donating group
OR Stronger EWG (resonance stabilized always wins)
The general steps do the aldehyde/ketone mechanisms follow.
Acid: Protonation --> Nucleophilic Attack --> Deprotonation
Base: Deprotonation --> Nucleophilic Attack --> Protonation
Identify the nucleophile, base, and electrophile in the following mechanism:

Green is nucleophile in first step.
Water is base in second step.
Carbonyl carbon behaves as the electrophile
A weakly deactivating O/P director
Chloro group
In order to add a chloro group Ortho to methyl group on toluene with a good yield what sequence of reactions would we use.
1) Sulfonation
2)Chlorination
3)treat with hot acid (remove sulfonyl group)
Relative to acetone, the following molecule has a higher or lower Khyd.
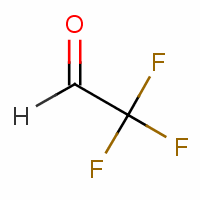
Khyd would be higher (products over reactants for K).Hydrate formation is favorable due to lack of electron donating alkyl groups and a EWG (trifluoromethyl).

Draw the first mechanistic step for the hydrolysis of an acetal under acidic conditions:

First Step sees protonation of acetal
Draw out a nucleophile and describe why it is nucleophilic