Particles smaller than an atom are called _______ particles.
Subatomic Particles
How many protons, electrons, and neutrons are in this element: 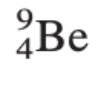
Protons and electrons: 4
Neutrons: 5
Energy levels
A unit of measurement that represents a very large quantity in chemistry
Mole
Name the student who is no longer with us :(
Chelseay
List the subatomic particles and their charges
Proton (positive)
Neutron (neutral)
Electron (negative)
This element has three energy levels and 15 protons
Phosphorus
When an electron falls from an excited state back to its ground state, it emits these particles
Photons
The number of atoms in 1 mole of a substance--list the number AND the name
6.022 x 10^23 (Avogadro's Number)
Bryer
The Cathode Ray Tube and a magnet discovered the charge of which subatomic particle?
Electron
This element has 6 energy levels and 107 neutrons
Tantalum
How many grams does 1 mole of Carbon weigh?
12 grams
It was a close race in our mole calculation lab. Which team came in second?
Trey and Kaige
Protons and Neutrons are found in which part of the atom?
Nucleus
This element has 19 electrons
Potassium
The maximum number of electrons that can be found on any orbital
2
How many grams does 6.022x10^23 atoms of Scandium weight?
Approximately 45
Phrase Colt responded with when asked "what time is it?"
Using Coulomb's Law, explain why the nucleus of an atom SHOULD split apart
The closer charged objects are, the stronger their attraction/repulsion. Protons all have positive charges, should strongly repel.
This element has an electron configuration of: 1s2, 2s2, 2p6, 3s1
Sodium
If an atom has the maximum possible number of 'f' orbitals in an energy level, how many electrons will occupy those 'f' orbitals?
14
How many atoms are in 2 moles of Nitrogen?
12.044 x 10^23 atoms
In a quote spoken by Cameron yesterday, it was said that "Wallinger's don't ________". What don't Wallingers do?
Cuss
The row number on the periodic table tells us the number of __________ an atom will have.
Energy Levels
The only element that does not have any neutrons.
Hydrogen
At most, a single energy level can have how many 's' orbitals?
1
How many grams does 100 moles of Hydrogen weigh?
100 grams
Which student made the claim that they "never eavesdrop"
Cameron