What are the 4 physical properties of matter?
Hardness, color, conductivity, malleability.
What is an atom?
An atom is the smallest particle of an element that retains its identity in a chemical reaction.
In the present periodic table, how are the elements organized?
They are organized by their atomic number.
What is a valence electron?
They are the electrons in the highest occupied energy level of an element’s atoms.
What is Avogadro's number?
It is the ratio between 1 mole and 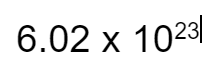
particles.
Define a gas.
Matter in which both the volume and shape are indefinite.
Who conducted the golf-foil experiment?
Rutherford
What side of the periodic table are the elements most metallic?
The left side.
In the periodic table, where can I find the number of valence electrons an atom has?
The element's group number.
How many moles are in 23 moles of oxygen?
1.38 molecules of oxygen
What are four types of chemical changes?
1. Transfer of energy
2. Production of gas
3. Color change
4. Formation of precipitate.
What was Rutherford's model of the atom?
What is the ionization energy?
It is the energy required to remove an electron from an atom.
What is the octet rule?
The octet rule states that in forming compounds, atoms tend to achieve the electron configuration of a noble gas.
What is the molar mass of 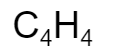
The molar mass is 52g.
What does the law of conservation of matter state?
Matter is neither created nor destroyed.
Explain Rutherford's experiment.
Rutherford put a gold foil in the middle of a metal boundary and he shot the gold foil with positively charged alpha particles. He noticed that the alpha particles never hit the middle of the gold foil, but they would hit at specific points far from the middle of the gold- foil.
What is the trend for the ionization energy as we move across the periodic table?
The ionization energy increases.
True or false: Anions and cations have opposite charges, which is why they do not attract.
False, they attract because they have opposite charges and opposites attract.
If I have 54 grams of helium and 20 grams of oxygen, what is the percent composition of helium?
73% composition of helium.
How do the particles in gas act?
They are far from each other. They do not want to touch each other.
What were Rutherford's three findings about the atom?
1. The atom is made of mostly empty space and electrons make up the volume of the atom.
2. The protons and neutrons are located in the center of the atom.
3. Protons and neutrons account for the mass of the atom.
What is the trend of the electronegativity as we move across the periodic table?
The electonegativity increases.
What is the electron configuration of oxygen?
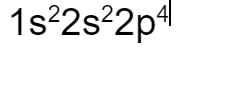
Define what happens in a decomposition chemical reaction.
A compound breaks down into elements.