An acid and a base react to form what?
What is water and a salt
How many valence electrons does Nitrogen have?
What is 5
Mr Campbell drank 3 cups of Lemonade (each cup is 240mL). How many liters did he drink?
What is 0.72L
Balance the following Reaction type
________Zn +_____ I2 → ZnI2
1,1
Given an actual yield of 5.2 grams and a theoretical yield of 5.7 grams, what would be the percent yield?
What is 91%
What would be the net ionic equation for the following equation?
Cu(NO3)2(aq) + Na2S (aq) → CuS (s)+ 2NaNO3 (aq)
Cu2+(aq) + S2- (aq) → CuS (s)
:max_bytes(150000):strip_icc():format(webp)/flavonol-58b5f2373df78cdcd816fecf.png)
What is the chemical formula for flavanol?
What is C15H10O3
How many atoms would be in 0.13 moles of Lithium (Li # 3)?
7.827x1022 atoms
What coefficients would be needed to balance the following reaction and what type of reaction is it?
N2 + H2 ----> NH3
What is 1,3,2
synthesis/ combination
Mr Campbell measured out 14 grams of sugar and added it to 400mL of water and 120mL of lemon juice to make lemonade, what was his molarity?
0.0787M
What are the products formed in the following reaction between phosphoric acid and a barium hydroxide, be sure to balance?
2H3PO4 + 3Ba(OH)2
What is 6H2O + Ba3(PO4)2
A strong acid and a strong base full disassociated in water, OH- and H+ were formed. Give the proper names for these ions.
What is hydroxide and hydronium (proton)
What would be the concentration of a 0.25L aqueous solution containing 4 grams of NaOH?
What is 0.4M
What type of reaction is this?
Na2SO3 + 2HCl →2NaCl + H2O + SO2
What is double displacement
When solid potassium chlorate (KClO3) decomposes, it produces solid potassium chloride (KCl) and oxygen gas (O2). If 50.0 grams of potassium chlorate decomposes completely, how many grams of oxygen gas are produced?
Balanced equation for the decomposition of potassium chlorate: 2𝐾𝐶𝑙𝑂3→2𝐾𝐶𝑙+3O2
19.58 grams
An _______is a substance that, when dissolved in water, increases the concentration of H+ ions.
What is an acid
What would be the molecular geometry shape formed by NCl3
What is trigonal pyramidal
Which of the following correctly defines a buffer?
- A buffer is a substance that increases the pH of a solution by releasing hydrogen ions.
- A buffer is a solution that prevents any change in pH upon the addition of an acid or a base.
- A buffer is a compound that reacts with water to form a weak acid or base, thus stabilizing the pH of a solution.
- A buffer is a solution that resists changes in pH when small amounts of acid or base are added to it. It consists of a weak acid and its conjugate base, or a weak base and its conjugate acid, which react with added H⁺ or OH⁻ ions to minimize changes in pH.
What is #4
Fill in the blanks
A _________ _________is a type of chemical bond formed by the sharing of one or more pairs of electrons between two atoms. This sharing occurs between a _________ and a non-metal.
What is a covalent bond, non-metal
Given the following reaction and told that 19.58 grams of O2 are produced, What would be the volume of gas produced assuming a standard temp of 295K and pressure of 1.01 atm.
R= 0.0821 L*atm/mol*K
2𝐾𝐶𝑙𝑂3→2𝐾𝐶𝑙+3O2
What is 14.97L
A solution is created by measuring 6.12x10-4 moles of NaOH and 8.2 x 10-5 moles of HCl into a container and then water is added until the final volume is 1L. What is the pH of this solution?
What is 10.7
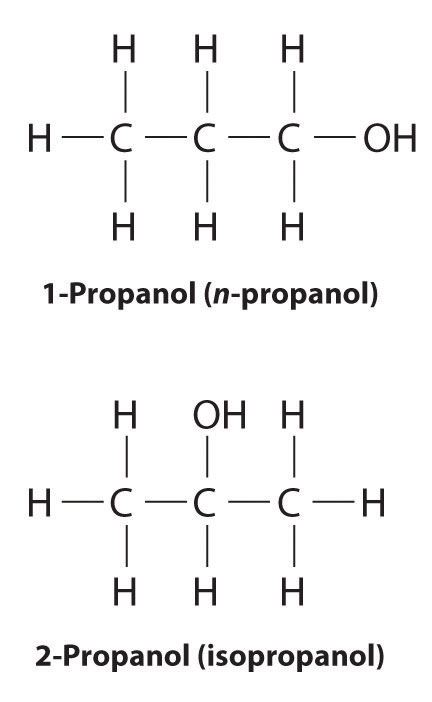
The following are examples of what?
What is Isomers
How many grams of H2O will be produced from 15 grams of C3H8 and 7 grams of O2?
C3H8 + 5O2 → 3CO2 + 4H2O
What is 3.17g H2O
What would be the cations and anions in the balanced reaction when they dissociate prior to forming products?
NH4OH + HI -------> H2O + NH4I
NH4+
OH-
H+
I-
Mr Campbell would like to dilute 240 mLs of his lemonade from 0.0787M to 0.025M. How many mLs of water does he need to achieve this?
What is 515.52mL