The color yellow falls under what category on the electromagnetic spectrum
What do these figures mean when using light equations
E, V, λ
what is: energy, frequency, and wavelength.
photoelectric effect is
what is, a process where electrically charged particles are released from or within a material when it absorbs electromagnetic radiation
this is how many electrons are in the P-orbital level
what are 6 electrons?
what is electronegativity?
what is measure of force and atom will excerpt on an electron during a chemical bond.
which color on the visible light spectrum has the longest wavelength, which color has the shortest wavelength?
What are Red and Violet?
what is the equation for Planck's constant
6.626 × 10-34 m2 kg / s
ejected electrons are called
what are photoelectrons.
what element does this electron configuration bring you too?
1s2 2s2 2p6 3s2 3p6 4s2
what is calcium?
what element on the periodic table has the highest electronegativity
what is Fluorine?
when the wavelength increases and and energy decreases, this is an example of what kind of relationship?
what is an inverse relationship?
what is the frequency of a light with a wavelength of 400 nm?
What is 7.5 x 1014 hz
name an everyday application of photoelectric effect
Photocopiers, electronics, solar panels.
what are 8 electrons, what are noble gasses
which element has a higher ionization energy; calcium or oxygen
What is oxygen?
Given the picture of a wave, what are the names of the highest and lowest points, respectively?

what are: the crest and the trough?
what is the equation for the speed of light
c = 2.998 x 108 ms-1
explain what is happening in this picture 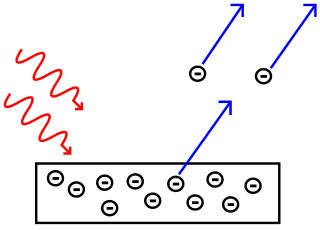
what islight waves hitting a surface (metal) and electrons being objected from that suface.
write the noble gas notation for this element

[Ne] 3s² 3p²
what is: increasing from left to right, and decreasing top to bottom.
In order name the 7 categories on the electromagnetic spectrum. From lowest frequency to highest frequency.
what are: Radio waves, micro waves, infrared waves, visible light, ultra violet waves, x-rays, gamma rays
caculate the wavelength given the frequency is 6.1x1014 Hz
λ = 4.92x10-7
what scientist discovered the photoelectric effect in connection with their work on radio waves
Heinrich Rudolf Hertz
write out the electron configuration for Ag
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d9
Arrange the following elements in order of increasing electronegativity; Hg, Xe, Mn
what is Xe, Mn, Hg.