How many chemical changes are listed below?
a. rusting of a metal can
b. boiling a cup of water
c. crushing an aspirin
d. digesting a candy bar
e. exploding of nitroglycerin
3 - a, d, e
Clyde Clumsy was directed to weigh a 500.0 g mass on the balance. After diligently goofing off for ten minutes, he quickly weighed the object and reported 458.0 g. Find the percent error involved.
The image below shows the absorption spectrum of helium. Of the labeled lines, which corresponds to the largest electron energy transition?
Line A
In which country are the Dolomites?
:max_bytes(150000):strip_icc()/header-DOLOMITESTG0422-fbe3eee35f6347f895af184866d0523d.jpg)
Italy
In class, you were given a metal sample with an unknown identity. What property was measured to determine the identity of the metal? Is this an intensive or extensive property?

The density, which is an intensive property, of the metal was found
Classify each as a pure substance or a mixture. If a mixture, indicate whether it is homogeneous or heterogeneous.
a. rice pudding 
b. magnesium
a. heterogeneous mixture
b. pure substance
How many liters of wine can be held in a wine barrel whose capacity is 31 gal?

1.2 x 102 L
Which pair of symbols represents nuclei that have the same number of neutrons?
a. 5626Fe and 5828Ni
b. 5826Fe and 5626Fe2+
c. 5727Co and 5728Ni
d. 5728Ni and 5828Ni
A.
Both have 30 neutrons
In which country would you find the Batu Caves?

Malaysia
In class, we learned about a neighborhood with high rates of lung cancer. We later learned that radon was the primary cause of the lung cancer cases. What specific process (just the name) does radon undergo to cause lung cancer?
Alpha decay
What type of quantity (for example, length, volume, density) do the following units indicate?
a. cm2
b. mg/L
c. nm
d. K
a. area
b. density
c. length
d. temperature
As an instructor is preparing for an experiment, he requires 225 g phosphoric acid. The only container readily available is a 150-mL Erlenmeyer flask. Is it large enough to contain the acid, the density of which is 1.83 g/mL? Show your work.
Yes, because the acid's volume will be 122.95 mL
Considering the visible portion of the electromagnetic spectrum only, which statement is true? Select all true statements.
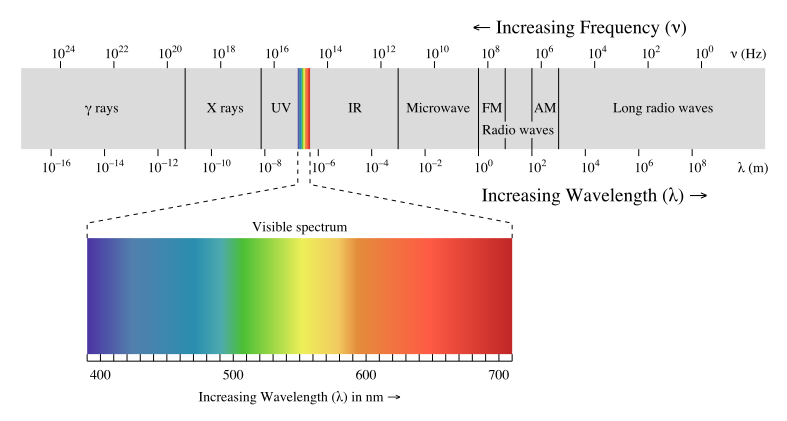
a. The wavelength of green is larger than that of purple light
b. The frequency of red is smaller than that of blue light
c. The energy of purple is greater than that of orange light
d. The speed of purple is greater than that of green light
A, B, and C
In which country is the Mona Lisa painting?

France
We wanted to know who killed our dear friend, Atom Davictim. What analytical chemistry technique were we understanding by completing this activity?
mass spectrometry
Three cubes of equal mass are composed of gold (density = 19.32 g/cm3), platinum (density = 21.45 g/cm3), and lead (density = 11.35 g/cm3). List the cubes from smallest to largest.
platinum, gold, lead
The recommended adult dose of Elixophyllin, a drug used to treat asthma, is 6 mg/kg of body mass. Calculate the dose in milligrams for a 185 pound person.

5 x 102 mg
Which of the following is a false statement about the photoelectric effect?
a. Electrons will not be ejected from a metal unless light of a certain energy or higher shines on it
b. When enough photons are absorbed by the metal, an electron will be ejected from the surface
c. A single photon can eject an electron if it has enough energy
d. A dim light can eject an electron from a metal as long as the light’s frequency is high enough
b. When enough photons are absorbed by the metal, an electron will be ejected from the surface
In which country would you find the Gardens by the Bay?

Singapore
In class, you had an open system with vinegar and baking soda and a closed system with vinegar and baking soda. Both systems were placed on the scale to measure initial mass and final mass. What process was being observed here?
law of conservation of mass
When performing the separating a mixture lab, you used the following equipment. Name each piece of equipment. What was being separate in this part of the lab?

tripod, wire gauze, alcohol burner
salt and water
Challenge Question: Gold can be hammered into extremely thin sheets called gold leaf. An architect wants to cover a 100 x 82 ft ceiling with gold leaf that is five-millionths of an inch thick. The density of gold is 19.32 g/cm3, and gold costs $953 per troy ounce. How much will it cost the architect to buy the necessary gold?
1 troy ounce = 31.1034768 g
12 inches = 1 foot
$6 x 10^4
It takes 7.21 x 10-19 J of energy to remove an electron from an iron atom. Would light with a wavelength of 3.21 x 10-2 m be able to remove an electron from an iron atom? Explain.
No, the maximum wavelength of light that can remove an electron is 2.76 x 10-7 m and this wavelength is longer, meaning the frequency and energy will not be great enough
In which country would you find the Appalachian Mountains?

United States
In class, you used a red laser pointer and a green laser pointer and observed what happened when shined on a pink object. You observed a color change when the green light shined on the pink, but no matter how bright the red light, you did not observe a color change. What was this demonstrating?
light's particle nature

