According to which theory or law is a chemical reaction most likely to occur when two particles with the proper energy and orientation interact with each other?
(1) atomic theory
(2) collision theory
(3) combined gas law
(4) law of conservation of matter
(2) collision theory
Which substance is an Arrhenius acid?
(1) H2
(2) HCl
(3) KCl
(4) NH3
(2) HCl
The three nuclides, U-233, U-235, and U-238, are isotopes of uranium because they have the same number of protons per atom and
(1) the same number of electrons per atom
(2) the same number of neutrons per atom
(3) a different number of electrons per atom
(4) a different number of neutrons per atom
(4) a different number of neutrons per atom
What is the number of moles of CO2 in a 220.-gram sample of CO2 (gram-formula mass 44 g/mol)?
(1) 0.20 mol
(2) 5.0 mol
(3) 15 mol
(4) 44 mol
(2) 5.0 mol
Which numerical setup can be used to calculate
the heat energy required to completely melt
100. grams of H2O(s) at 0°C?
(1) (100. g)(334 J/g)
(2) (100. g)(2260 J/g)
(3) (100. g)(4.18 J/g•K)(0°C)
(4) (100. g)(4.18 J/g•K)(273 K)
(1) (100. g)(334 J/g)
Zn(s) + Co2+(aq) → Zn2+(aq) + Co(s)
Which statement describes the electrons involved in this reaction?
(1) Each Zn atom loses 2 electrons, and each
Co2+ ion gains 2 electrons.
(2) Each Zn atom loses 2 electrons, and each
Co2+ ion loses 2 electrons.
(3) Each Zn atom gains 2 electrons, and each Co2+ ion loses 2 electrons.
(4) Each Zn atom gains 2 electrons, and each
Co2+ ion gains 2 electrons.
(1) Each Zn atom loses 2 electrons, and each Co2+ ion gains 2 electrons.
Addition of a catalyst can speed up a reaction by providing an alternate reaction pathway that has a
(1) lower activation energy
(2) higher activation energy
(3) lower heat of reaction
(4) higher heat of reaction
(1) lower activation energy
Which statement describes an electrolyte?
(1) An electrolyte conducts an electric current as a solid and dissolves in water.
(2) An electrolyte conducts an electric current as a solid and does not dissolve in water.
(3) When an electrolyte dissolves in water, the resulting solution conducts an electric current.
(4) When an electrolyte dissolves in water, the
resulting solution does not conduct an
electric current.
(3) When an electrolyte dissolves in water, the resulting solution conducts an electric current.
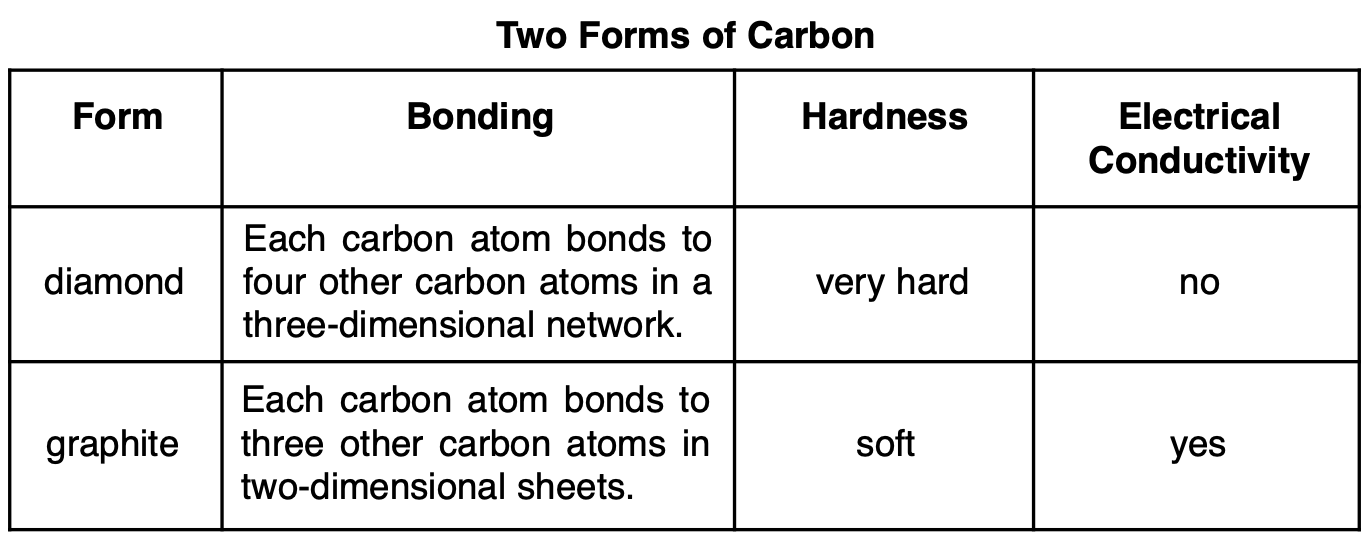
Diamond and graphite have different properties because they have different
(1) crystal structures
(2) electronegativities
(3) numbers of protons per atom
(4) numbers of valence electrons per atom
(1) crystal structures
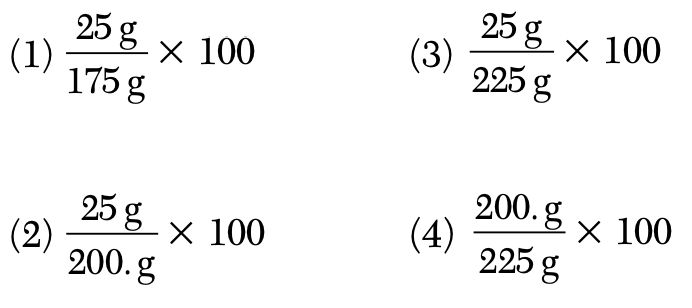
A solution contains 25 grams of KNO3 dissolved in 200. grams of H2O. Which numerical setup can be used to calculate the percent by mass of KNO3 in this solution?
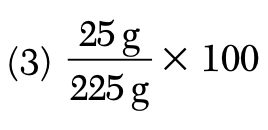
During which phase change does the entropy of a sample of H2O increase?
(1) H2O(g) → H2O(ℓ)
(2) H2O(g) → H2O(s)
(3) H2O(ℓ) → H2O(g)
(4) H2O(ℓ) → H2O(s)
(3) H2O(ℓ) → H2O(g)
What are the two oxidation states of nitrogen in NH4NO2?
(1) +3 and +5
(2) +3 and -5
(3) -3 and +3
(4) -3 and -3
(3) -3 and +3
Which compound is saturated?
(1) butane
(2) ethene
(3) heptene
(4) pentyne
(1) butane
Which type of reaction occurs when an Arrhenius acid reacts with an Arrhenius base to form a salt and water?
(1) combustion
(2) decomposition
(3) neutralization
(4) saponification
(3) neutralization
NaCl(aq) + AgNO3(aq) → NaNO3(aq) + AgCl(s)
This reaction is classified as a
(1) synthesis reaction
(2) decomposition reaction
(3) single replacement reaction
(4) double replacement reaction
(4) double replacement reaction
What is the molarity of 0.50 liter of an aqueous solution that contains 0.20 mole of NaOH (gram-formula mass = 40. g/mol)?
(1) 0.10 M
(2) 0.20 M
(3) 2.5 M
(4) 0.40 M
(4) 0.40 M
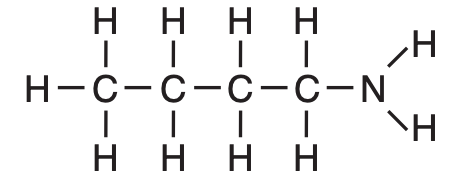
What is a chemical name for this compound?
(1) 1-butanamide
(2) 4-butanamide
(3) 1-butanamine
(4) 4-butanamine
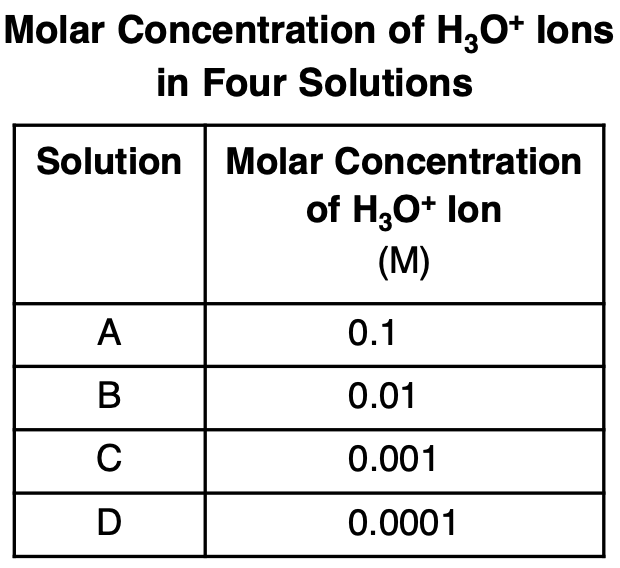
Which solution has the highest pH?
(1) A
(2) B
(3) C
(4) D
(4) D
An alcohol and an ether have the same molecular formula, C2H6O. These two compounds have
(1) the same functional group and the same physical and chemical properties
(2) the same functional group and different physical and chemical properties
(3) different functional groups and the same physical and chemical properties
(4) different functional groups and different
physical and chemical properties
(4) different functional groups and different physical and chemical properties
Compared to the energy released per mole of reactant during chemical reactions, the energy released per mole of reactant during nuclear reactions is
(1) much less
(2) much greater
(3) slightly less
(4) slightly greater
(2) much greater
What is the formula for iron(II) oxide?
(1) FeO
(2) FeO2
(3) Fe2O
(4) Fe2O3
(1) FeO
A mixture consists of ethanol and water. Some
properties of ethanol and water are given in the
table below.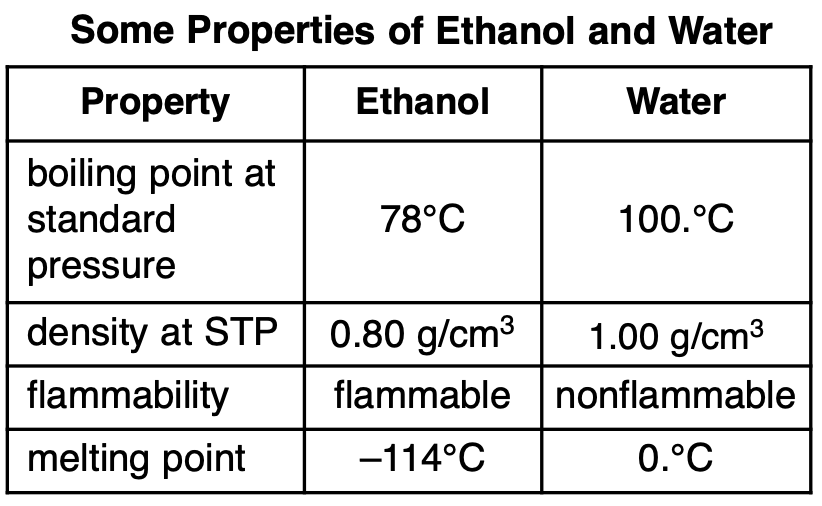
Which statement describes a property of ethanol after being separated from the mixture?
(1) Ethanol is nonflammable.
(2) Ethanol has a melting point of 0.°C.
(3) Ethanol has a density of 0.80 g/cm3 at STP.
(4) Ethanol has a boiling point of 89°C at
standard pressure.
(3) Ethanol has a density of 0.80 g/cm3 at STP.
C4H10 Cl2 → C4H9Cl HCl
Which type of reaction is represented by the equation?
(1) addition
(2) substitution
(3) fermentation
(4) polymerization
(2) substitution
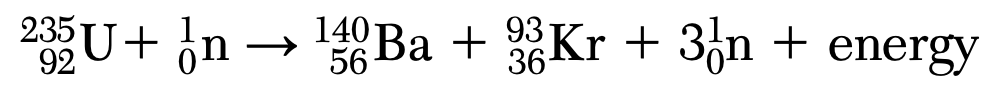
Which type of nuclear reaction is represented by the equation?
(1) fission
(2) fusion
(3) beta decay
(4) alpha decay
(1) fission
Which metal is most easily oxidized?
(1) Ag
(2) Co
(3) Cu
(4) Mg
(4) Mg
Which phrase describes a risk of using the radioisotope Co-60 in treating cancer?
(1) production of acid rain
(2) production of greenhouse gases
(3) increased biological exposure
(4) increased ozone depletion
(3) increased biological exposure
2KClO3(s) → 2KCl(s) + 3O2(g)
How many moles of KClO3 must completely react to produce 6 moles of O2?
(1) 1 mole
(2) 2 moles
(3) 6 moles
(4) 4 moles
(4) 4 moles
A rigid cylinder with a movable piston contains a sample of hydrogen gas. At 330. K, this sample has a pressure of 150. kPa and a volume of 3.50 L. What is the volume of this sample at STP?
(1) 0.233 L
(2) 1.96 L
(3) 4.29 L
(4) 6.26 L
(3) 4.29 L
Which half-reaction equation represents
reduction?
(1) Cu → Cu2+ + 2e-
(2) Cu2+ + 2e-? → Cu
(3) Ag + e-? → Ag+
(4) Ag+ → Ag + e-?
(2) Cu2+ + 2e-? → Cu
Which nuclear emission has the least penetrating power and the greatest ionizing ability?
(1) alpha particle
(2) beta particle
(3) gamma ray
(4) positron
(1) alpha particle