Rusting is an example of a physical or chemical change?
What is a Chemical change
Copper (Cu) has an atomic number of 29 and an atomic mass of 63.5 amu. How many protons does copper have?
What is 29.
What are the three states of matter?
What are: solid, liquid and gas.
What is the weight of a single electron?
What is 0 amu?
Hydrogen has an atomic number of 1 and an atomic mass of 2 amu. How many neutrons does one atom of hydrogen have?
What is 1 neutron.
Ice melting is an example of what kind of change?
What is, a physical change?
Where can you find protons in an atom?
What is The Nucleus?
If you have a liquid substance, and you increase the temperature, but decrease the pressure, what state will your substance turn into?
What is gas?
Where are neutrons found in an atom and what is their weight?
What is in the nucleus and 1 amu?
List protons, neutrons, and electrons for: ![]()
What is :
5 Electrons
5 Protons
6 Neutrons
What is:
A change in which the physical properties of a substance are altered. Usually reversible.
What is the weight of a single proton?
What is 1 amu?
At what point can all three states exist at one time?
What is the triple point?
The number of protons and/or the number of electrons?
List 4 Physical properties.
What are: Shape, Form,Taste,Hardness,Texture, Color,State(solid, liquid or gas)?
Provide the definition of a chemical change.
What is:
A change of materials into another, new materials with different properties and one or more than one new substances are formed. Usually irreversible.
If an atom has 6 electrons and 7 neutrons, how many protons does it have?
What is 6 electrons?
What state is this a picture of?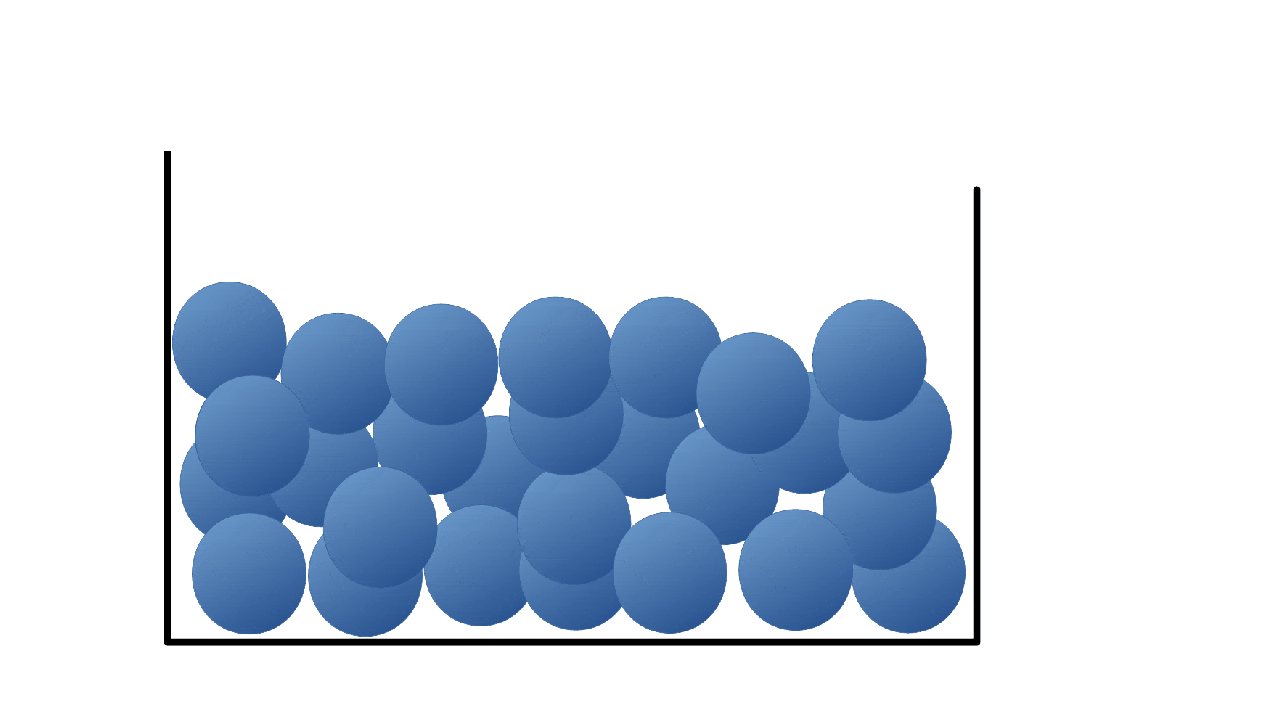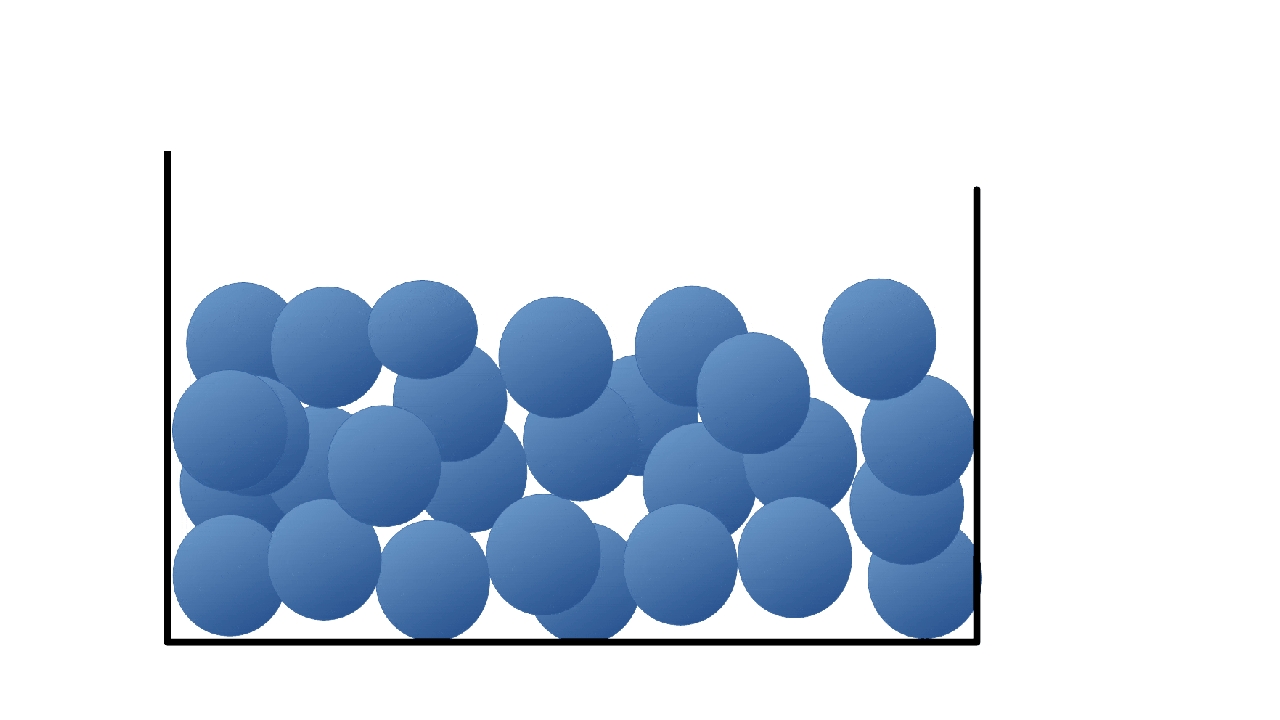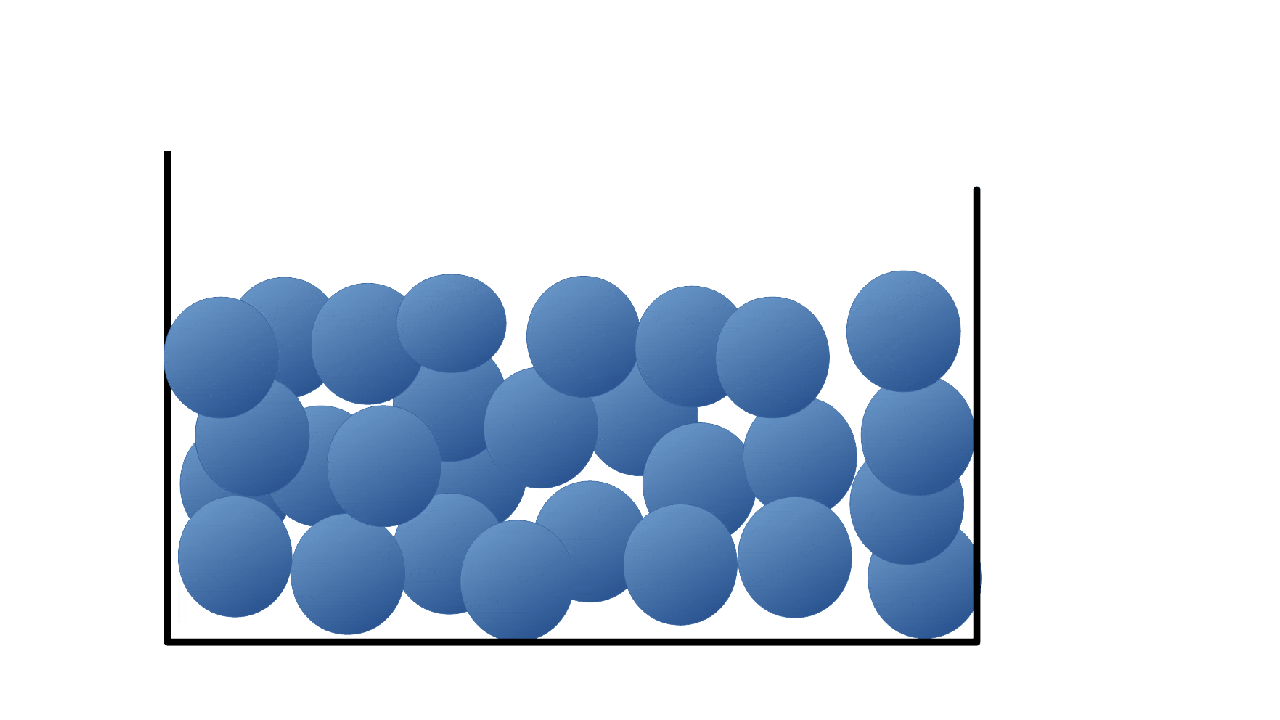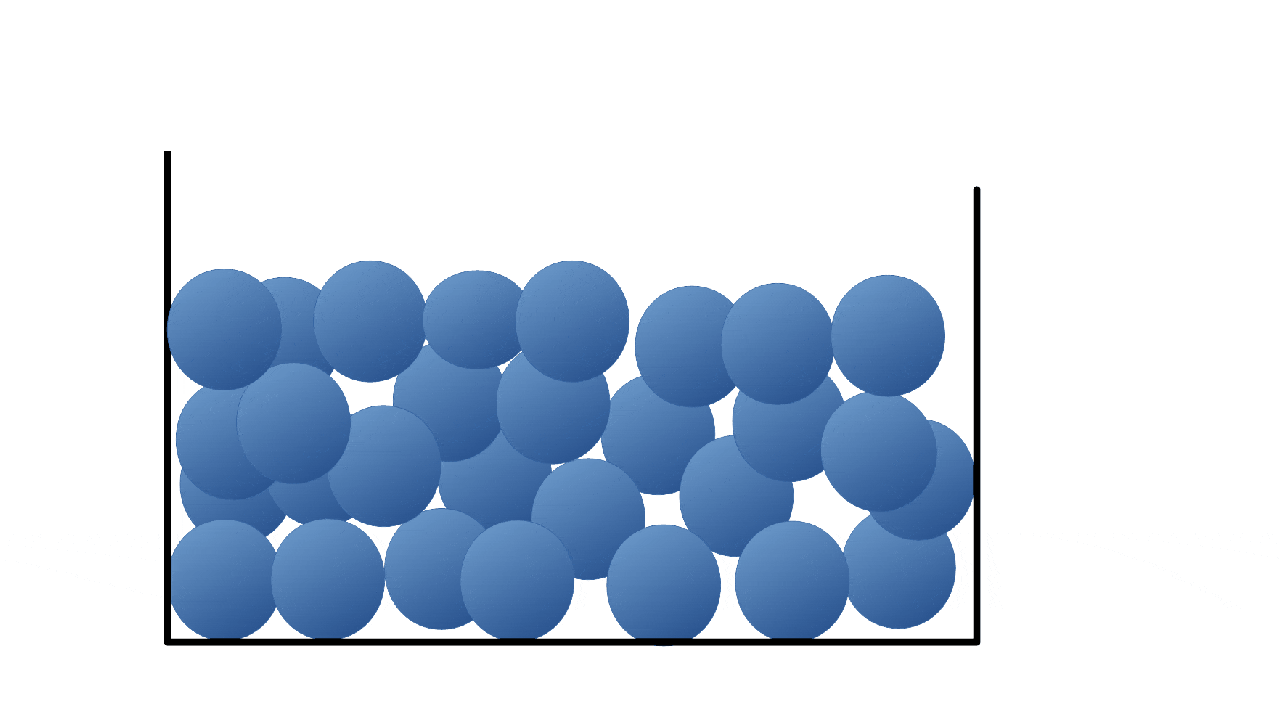
What is liquid?
Why does the number of protons and electrons within an atom need to be the same?
What is, because an atom needs to have an equal charge in order to be stable?
List protons, neutrons and electrons for:

What is:
109 electrons
109 protons
167 neutrons
Mixing salt and water until the salt dissolves. What kind of change is this an example of? For actually points, explain why.
What is:
A chemical change. There are bonds broken and reformed on an atomic level forming a new product or substance when salt is dissolved in the water.
If an atom has an atomic mass of 16 amu and 9 neutrons, how many protons does this atom have?
What is 7 atoms?
What is the critical point?
What is, where a new phase called supercritical fluid forms. A state that is both liquid and gas.
What is:
4 electrons
5 neutrons
4 protons
List at least 4 chemical properties.
What are: Burning or flammability, Rusting, Tarnishing, odor?