Which reaction released most energy?
A) 450 KJ/mol
B) -250 KJ/Mol
C) -650 KJ/mol
Reaction C
Which of the following molecules is the most polar?
a) CH4
b) CH3Cl
c) H2O
H2O
Which type of bond will have the greatest average bond enthalpy
a) C-C
b) C=C
C=C
Which molecule is most likely to dissolve in water?
a) C2H6
b) C2H5Cl
c) C2H5O
C2H5O
How many moles of Hydrogen gas are there at 25 degrees, 101.35kpa if it has a volume of 35L.
1.43mol
Why does batman like sodium?
Na Na Na Na Na Na Na BATMAN!
Determine the number of moles in of 7g of He2
1.75mol
Rank the intermolecular forces from weakest to strongest
Dispersion forces, Dipole-Dipole, Hydrogen bonding
How does a catalyst speed up the rate of a reaction?
A catalyst provides an alternative pathway for the reaction to take place, reducing the activation energy.
Is the following product soluble or insoluble?
Fe+2H2SO4→2FeSO4+H2
Soluble
Write the electron configuration for potassium
1s2 2s2 2p6 3s2 3p6 4s1
What volume of 0.0995M Al(NO3)3 will react with 3.66 g of Ag according to the following chemical equation?
3Ag(s)+Al(NO3)3(aq)→ 3AgNO3+Al(s)
V = n/c = 0.0113/0.0995 = 0.114L
What would be the enthalpy change if 4 moles of nitric oxide (NO) reacted with 2 moles of oxygen?
2NO(g) + O2(g) à 2NO2(g) ΔH = -114 kJ/mol
ΔH = -228 kJ/mol
Propose a seperation technique to seperate water and Oil.
Decant the oil, since it will be floating on top of the water.
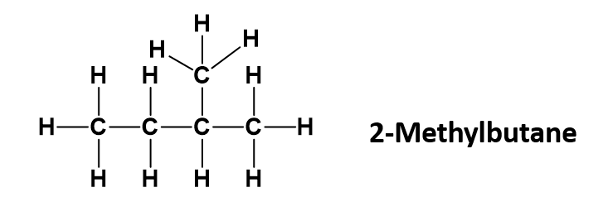
Draw 2-methyl Butane
Propose a separation technique to seperate ethanol and water.
Distillation, ethanol has a lower boiling point than water.
Determine the enthalpy for the following reaction:
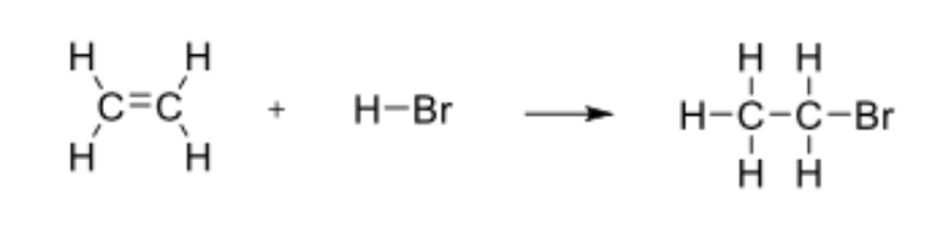
ΔH = -65kJ/mol
Use the graph to determine the amount of KNO3 that precipitates out of solution if 100g is dissolved at 70 degrees and the temperature is brought down to 40 degrees.
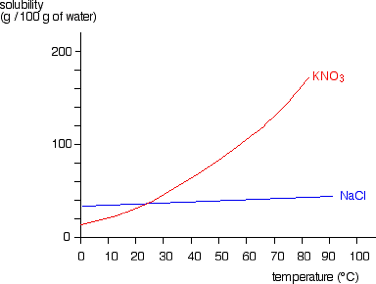
100-61 = 39g of KNO3
What is the shape of BF3
Trigonal Planar
Which of these molecules is less likely to be soluble in water?

Which of the following molecules has no net dipole moment?
a)HCl
b)H2O
c)CCl4
d)CH3Cl
CCl4
What shape and what intermolecular forces does methoxymethane (CH3OCH3) experience?
Bent shape, dipole-dipole and dispersion forces.
1.A mixture of methane and methanol is put through a chromatography column with a polar stationary phase and a non-polar mobile phase. The chromatogram is on the right. Which peak is most likely to be methanol? JUSTIFY
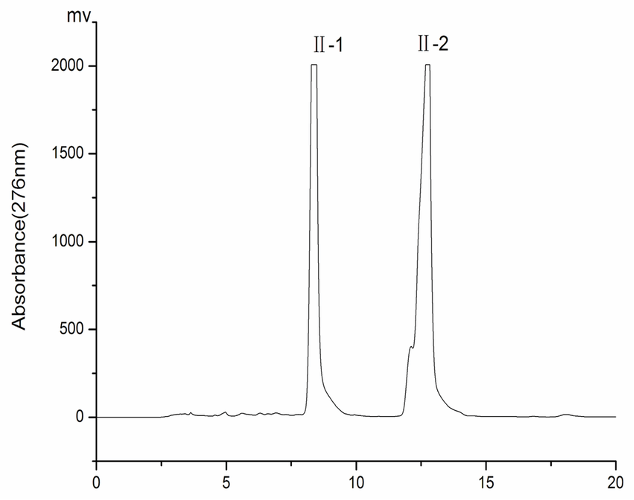
Second peak is methanol due to it being polar, and will interact with the polar stationary phase for longer periods of time. Therefore it will take longer to pass through the column.
Write out the overall chemical equation, and net ionic equation of the following reaction.
Li2SO4 + BaCl2 ->
Li2SO4(aq) + BaCl2(aq) -> 2LiCl(aq) + BaSO4(s)
SO42-(aq) + Ba2+(aq) -> BaSO4(s)
Aluminium metal reacts with Hydrogen bromide to produce aluminium bromide and hydrogen gas. If 87 grams of Al reacts with 401 grams of HBr.
If 4.68 g of hydrogen gas are formed experimentally. Calculate the percentage yield.
% yield = (4.68/5.01) x 100 = 93.41 %
Given the following equations and ∆H values, determine the enthalpy change (kJ/mol) at 298K for the reaction:
2OF2 + 2S -> SO2 + SF4
OF2 + H2O -> O2 + 2HF ∆H = -276.6 kJ/mol
SF4 + 2H2O -> 4HF + SO2 ∆H = -827.5 kJ/mol
S + O2 -> SO2 ∆H = -296.9 kJ/mol
∆H = -553.2 + 827 + -593.8
= -320 kJ/mol
20.83 g of a diatomic gas occupies 4.104 L at 79.97 kPa at 30.0 °C. What is its molecular mass?
What is the identity of this gas?
Br2
What is the empirical formula of a compound composed of 21.2 % N, 6.1 % H, 24.2 % S and 48.5 % O ?
N2H8SO4
Determine what precipitate (if any) will be produced when solutions of barium hydroxide (Ba(OH)2) and potassium sulfate (K2SO4) are added together.
Barium sulfate BaSO4
Devise a method to determine whether bromide, aluminium and calcium ions are present in an unknown sample. Present your method as numbered steps, detailing the equipment that could be used in a laboratory.
1. To determine the presence of bromide ions in the unknown solution, the solution can be reacted with silver nitrate (AgNO3) in a test tube.
2. Carbonate solution forms CaCO3 which is insoluble.
3. NaOH added to remove aluminium ions.