Define Element
A substance that cannot be broken down into simpler substances by chemical means.
What is a mixture
2 or more compounds
Ionic Bonds are formed between _________ and ___________
metals and non-metals
cations and anions
+ and -
Covalent Bonds are formed between 2 _____________
non-metals
Define a compound
2 or more elments chemically bonded together
2 compounds that do not mix well are called a _______________ mixture
Heterogeneous
In ionic bonds the electrons swap/share (choose 1)
swap
Covalent Bonds form when electrons are swapped/shared? (choose 1)
shared
Is chopping wood is an example of a physical or chemcial change?
Physical Change (Not changing chemical makeup)

Na+1 and Cl-1 make?
NaCl


I burn wood in my backyard to create fire. Is this an example of a chemical or physical change?
Chemical
A heterogeneous mixture is also called a ____________?
Solution
Solve and name the ionic bond between Mg and Br?
MgBr2
Magnesium Bromide
What are some properties of covalent bonds
Low melting points
Don't dissolve in H20
Non Conductive
Phase Changes are what kind of change
Physical!
Think Ice --> Water --> Steam (All H20)

In a salt water solution what is the solvent and what is the solute?
Water Solvent
NaCl Solute
Draw the valence electrons to represent Potassium Chloride
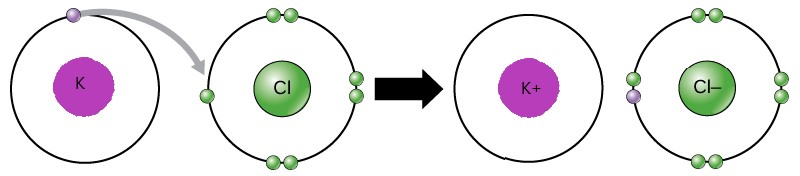
What is a bond?
a pair of electrons
can be single, double, or triple