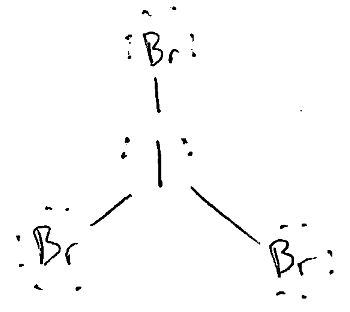
Draw the Lewis Dot Structure for IBr3.
What is the F-Si-F bond angle in SiF4?
Exactly 109.5o.
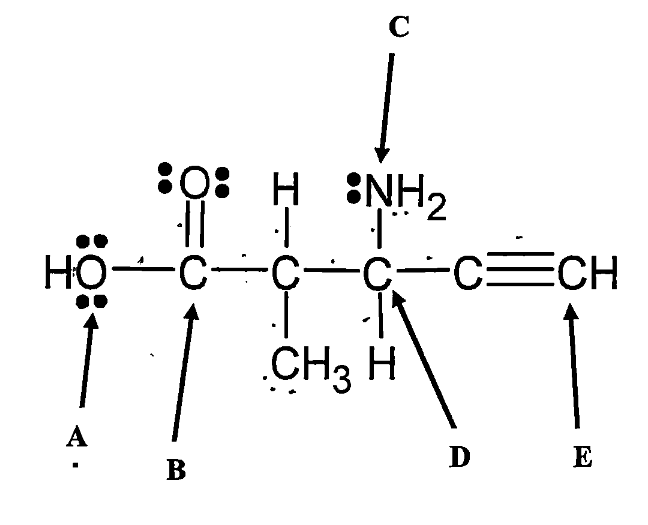
What is the hybridization of atom A?
What is the molecular shape around atom A?
sp3
bent
Draw the MO diagram for He2. Does it exist?
Bond order = 0, so it does not exist.
Balance the following reaction:
C6H12O6 + O2 --> CO2 + H2O
C6H12O6 + 6 O2 --> 6 CO2 + 6 H2O
Which is the best possible structure for N2O?
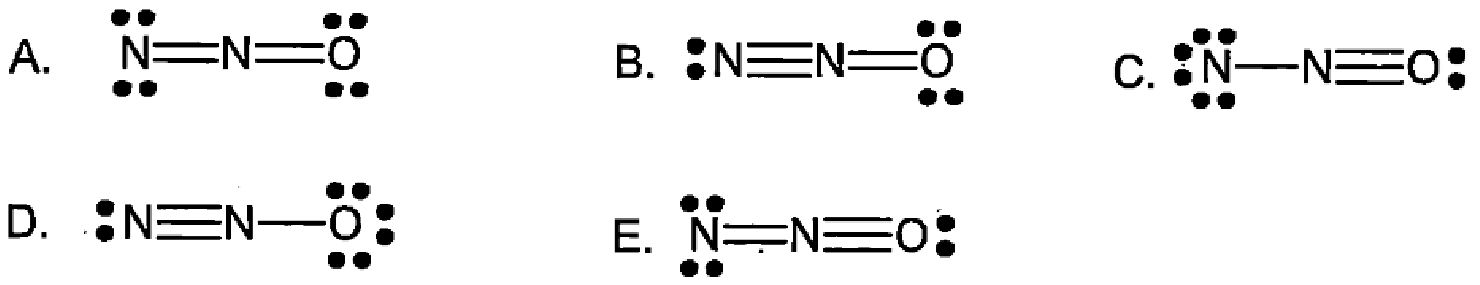
D.
What is the smallest Br-I-Br bond angle in IBr3?
less than 90o.

What is the hybridization of atom B?
What is the molecular shape around atom B?
sp2
trigonal planar
Which of the following is paramagnetic? Select all that are correct.
NO-, N2, Ne2+, C2+, F2
NO-, Ne2+, C2+
Match:
1. Combination Reaction
2. Decomposition Reaction
3. Combustion Reaction
a) C --> A + B
b) CxHyOz + O2 --> CO2 + H2O
c) A + B --> C
1c
2a
3b
What is the correct Lewis Dot Structure, ED and molecular shapes for ClO2-?
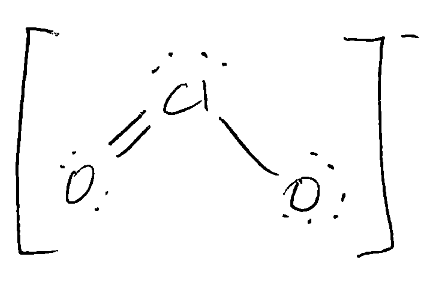
ED: tetrahedral
Molecular: Bent
How many sigma and pi bonds are present in this structure?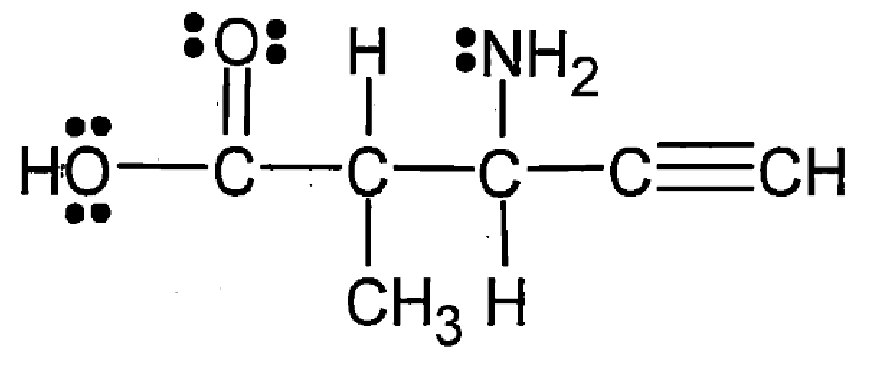
17 sigma, 3 pi

What is the hybridization of atom C?
What is the molecular shape around atom C?
sp3
trigonal pyramidal
Determine the bond order of each member of the following group, and determine which member is predicted by the molecular orbital model to have the strongest bond.
Li2, Be2+, Be2
Li2, bond order = 1
Be2+, bond order = 1/2
Be2, bond order = 0
strongest: Li2
How many grams of CO2 can 1.0g of LiOH absorb?
LiOH + CO2 --> Li2CO3 + H2O
0.92g
What is the correct Lewis Dot Structure, and molecular shape for ICl4+? Is it polar?
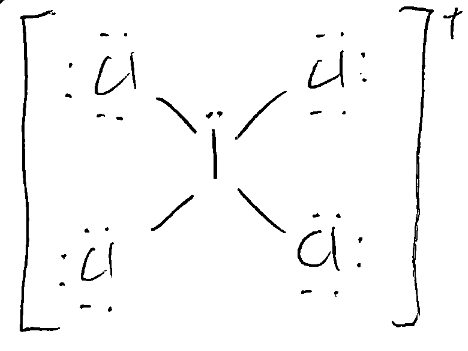
Molecular Shape: see-saw
Polarity: polar
What is the O-S-O bond angle in SO32-? (Hint: it can be an interval between two numbers.)
between 90o and 109.5o.

What is the hybridization of atom D?
What is the molecular shape around atom D?
sp3
tetrahedral
What is the bond order for CN-? Is it diamagnetic or paramagnetic?
bond order = 3
diamagnetic
4.58g of a compound containing carbon, hydrogen, oxygen, and nitrogen was combusted in excess oxygen, producing 7.80g of CO2, 2.39g of H2O, and 1.24g of N2. What is the empirical formula of the compound?
C6H9O2N3
Draw the Lewis Dot structures for the following compounds along with ANY AND ALL equivalent resonance structures that they have: NO+, NO-, NO2-, NO3-. Then answer the following questions:
a) Which compound has the strongest/weakest N-O bond?
b) Which compound has the longest/shortest N-O bond?
1) Triple bond and one lone pair on each, no resonance.
2) Double bond with two lone pairs on each, no resonance.
3) One double (2 l.p. on O) and one single (1 l.p. on O) bond, one lone pair on N, 1 resonance structure.
4) One double (2 l.p. on O) and two single (1 l.p. on O's) bonds, no lone pairs on N, 3 resonance structures.
a) strongest: NO+, weakest: NO3-
b) longest: NO3-, shortest: NO+
Which substance has a bond order of 3/2?
NO-, N2, Ne2+, C2+, F2
C2+

What is the hybridization of atom E?
What is the molecular shape around atom E?
sp
linear
Draw the MO diagram for NO. What is the bond order? Is it dia- or paramagnetic?

Bond order: 5/2
paramagnetic
How many grams of the precipitate are formed when 20.0ml of 1.35M FeCl3 reacts with 35.0ml of 1.65M AgNO3 in this reaction? Clearly identify the limiting reagent in this reaction. If you actually isolate 6.83g, what is the percent yield?
FeCl3(aq) + 3AgNO3(aq) -> 3AgCl(s) + Fe(NO3)3(aq)
LR: AgNO3
% yield: 82.5%