on a Break!
Hot in Here
Potables
bit of ..... Spice
Describe the size of gas particles.
What is very small compared to the distance between them?
These two variables relate inversely proportional to each other
What is pressure and volume?
31°C in Kelvin
What is 304 K?
The SI unit of pressure.
What is Pascal (Pa)?
The kind of collisions gas particles undergo.
What is elastic?
Describe the movement of gas particles.
What is constant, random motion?
The two variables that are directly proportional with temperature.
What is pressure and volume?
The reason we use the Kelvin scale.
There are no negative numbers?
The reason you shouldn't put cans of shaving cream in a fire.
What is particles move faster from increased temperature, have more collisions until the pressure bursts the container open?
The container with the lowest pressure (equal number of gas molecules in each).
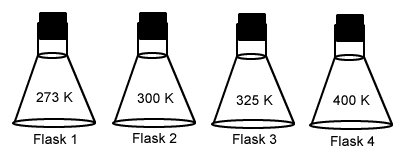
What is flask 1?
Describe the variables in this situation.
What is decreasing the volume causes pressure to increase?
The new volume of 2L of a gas if the pressure is decreased from 15 to 5.
What is 6L?
The lowest possible temperature (with units) and its special name.
What is Absolute Zero, 0K (-273°C)?
The reason your tire pressure is low on a chilly morning.
What is decreased temperature, particles moving slower, so fewer collisions and lower pressure.
This is what temperature is measuring.
What is the average kinetic energy?
The reason why gas is compressible.
What is there are large spaces between gas particles?
The pressure of a sealed container at 150kPa if the temperature is doubled from 100°C to 200°C.
What is 190 kPa?
100 K expressed in °C.
What is -173°C?
The reason pressure cookers can cook food faster.
What is the increased pressure increases the number of collisions so the food cooks faster.
In which flask of all same size, same temperature and same number of molecules are molecules moving fastest and why.
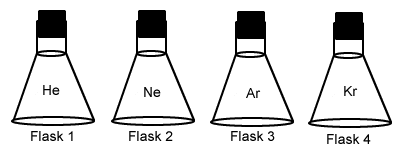
What is all flasks at same temperature, so KE is equal, therefore lightest particle moves fastest.
The formula that has initial and final pressure, volume, and temperature variables.
What is the Combined Gas Law?
The phenomenon that gas pressure measures.
What is collisions with the side of the container?
The physical phenomenon that occurs at -273°C.
What is all particle motion stops and volume of particles are zero.
Band that sings "Under Pressure".
Who is Queen?
The temperature and pressure conditions under which gases behave most ideally.
What is low pressure and high temperature?