This type of element is not likely to form a covalent bond
What is a metal?
NH3

sulfate ion
tetrahedral
The atom with a greater electronegativity is most likely to carry this type of formal charge
What is negative?
hybridization of a atom in a bent shape
sp2 or sp3
The type of bond formed between to atoms is dependent on this property
What is electronegativity?
XeF4
hybridization of Xe
sp3d2
hydrogen cyanide
linear
Draw the dipole moment for the H-Cl bond labeling partial charge

the number of sigma and pi bonds in the following molecule and the number of nonbonding electron pairs
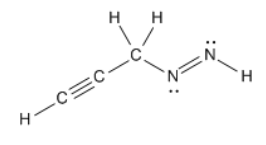
σ :8
π :3
nonbonding pairs: 2
Rank the following molecules from highest dissociation energy to lowest
C2H4 , C2H2 , C2H6
C2H2 > C2H4 > C2H6
ClO4-
hybridization of Cl
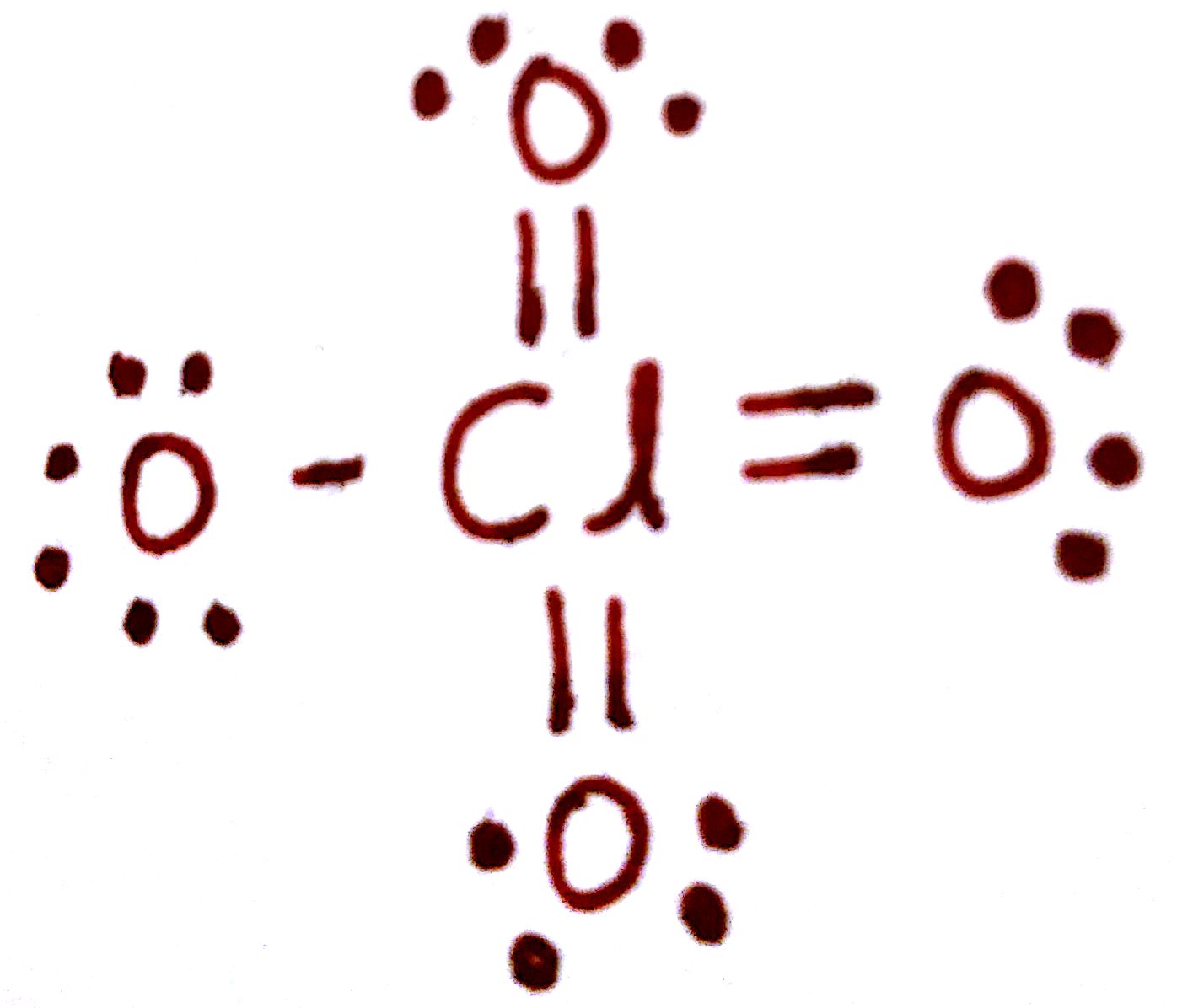
Need brackets and charge, minimize formal charge
Cl: sp3
sulfur hexafluoride
octahedral
polarity of sulfur trioxide
nonpolar

Calculate the formal charge on each atom in the phosphate ion
Rank the following molecules from lowest dissociation energy to highest
SO2 , SeH2 , O3
SeH2 < O3 < SO2
CH3CH2NH2
hybridization on each C&N

sp3 for all
sulfur difluoride
bent
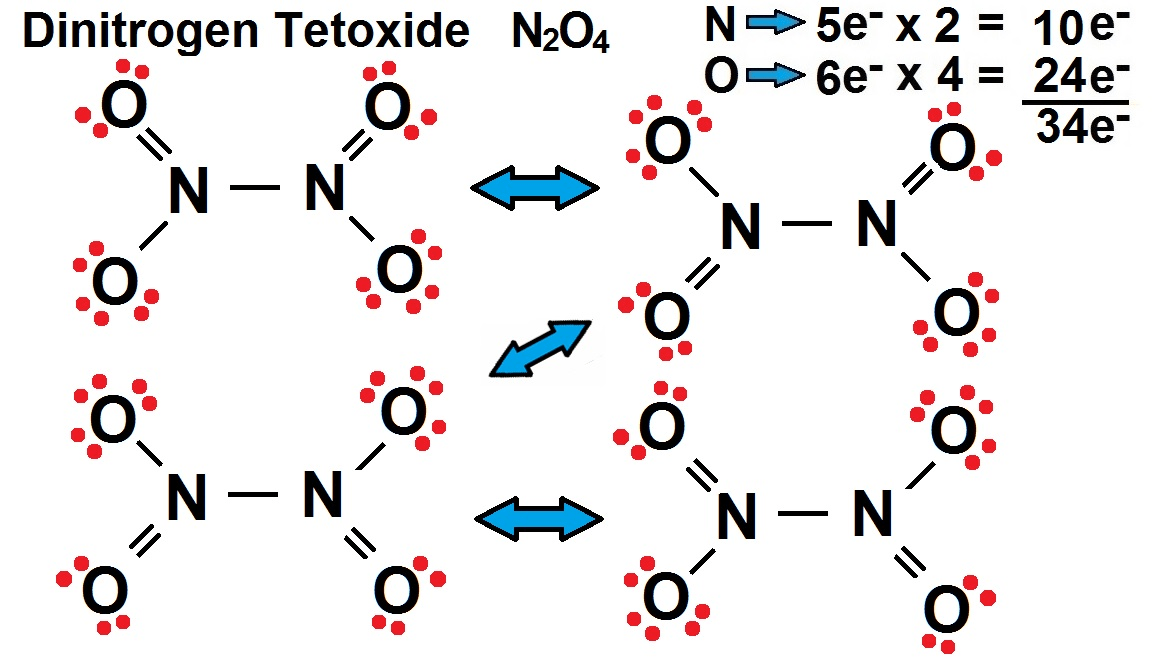
polarity of dinitrogen tetroxide
nonpolar- with resonance would be completely symmetrical
C2H4 + HBr --> C2H5Br
-57 kJ/mol
The ionic substance with the largest lattice energy
LiCl, GaP, CaO, & AlN
What is AlN (largest charges, +3 & -3, and smallest radii)?
CH3COOCH3
hybridization of each carbon

from left to right: sp3,sp2,sp3
selenium tetrafluoride
see-saw
The largest known living organism
Aspen Grove
