In a covalent bond, this type of bond is unequally sharing electrons.
What is polar bond?
The three rules used to organize electrons into their electron orbials.
What are the Aufbau principle, Hund's Rule, and the Pauli Exclusion Principle?
A bond that can form between two or more metals by sharing a cloud of electrons.
What is metallic bonding?
When these are present, the shape will be altered due to pushing against the bonds?
What are lone pairs or unbounded electrons on the center atom?
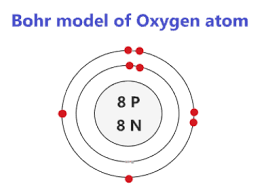
DAILY DOUBLE : Draw the Bohr model for Oxygen.
DAILY DOUBLE: ALL GROUPS DRAW THE LEWIS STRUCTURE FOR Na bonding with O.
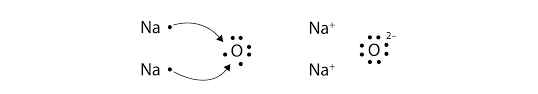 WHAT IS Na2O
WHAT IS Na2O
An arrow that shows the pull of electrons in a bond.
What is a dipole moment?
The element that has the electron configuration of 1s22s22p5
What is fluorine?
Ionic compounds can contain these covalently bonded ions.
What are polyatomics?
The number of valence electrons hydrogen needs in a Lewis structure?
What is TWO?
Polar or Nonpolar?
CO
What is Polar?
The element that has the following electron orbital diagram.
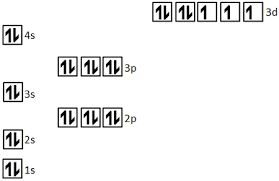
What is cobalt?
This is used when metals have more than one possible charge.
What are roman numerals?
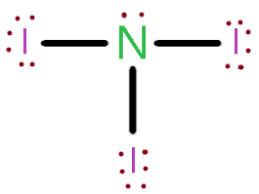
DAILY DOUBLE: ALL GROUPS ANSWER
This IS THE LEWIS STRUCTURE FOR NI3
Polar or Nonpolar:
SSe3
What is Nonpolar?
THE MOLECULAR GEOMETRY OF CBr4
What is tetrahedral
The trend that explains the balance between the positive nucleus and the outer electrons AND supports the reasoning behind all the other trends.
What is effective nuclear charge?