Every molecule of H2O contains this
What are two atoms of hydrogen and one atom of oxygen?
Fe3O2 - type of bond
What is ionic?
occurs with electrons in nonpolar covalent bonds
What are they are shared equally?
tend to be liquids or gases at room temperature
What are covalent compounds?
DAILY DOUBLE!
average distance between the nuclei of two bonded atoms
What is bond length?
an ion made of two or more nonmetal atoms
What is a polyatomic ion?
N2 - type of bond (be specific)
What is nonpolar covalent?
occurs with electrons in an ionic bond
What is transferred?
usually result in a compound with a high melting and boiling points
What are ionic compounds?
angle formed by two bonds to the same atom
What is a bond angle?
Type of molecular model
What is space-filled ?
HBr - type of bond (be specific)
What is polar covalent?
DAILY DOUBLE!
occurs with electrons within a polar covalent bond
What is shared unequally?
usually forms between atoms of oppositely charged ions
What are ionic bonds?
Lewis Dot diagram for bromine
What is  ?
?
Type of molecular model shown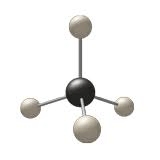
What is the ball and stick model?
KOH - type of bond
What is ionic?
The measure of the force an atom has to attract electrons
What is electronegativity?
result in compounds that are poor conductors
What are covalent compounds?
reason why atoms bond
What is they are most stable if their outermost energy level is full?
Type of model shown
What is a structural model?
Bond in which a "sea of electrons" results
What is a metallic bond?
charged atom
What is an ion?
What are ionic compounds?
The forces that hold different atoms or ions together
What are chemical bonds?