What type of electrons are involved in chemical bonds?
valence electrons
What is the oxidation number of phosphorus (P)?
P-3
Why is this the incorrect name for the compound shown?
NaO2 = sodium dioxide
The compound is ionic and should be named sodium oxide.
When writing the chemical formula for an ionic compound the (metal/nonmetal) is listed first and the (metal/nonmetal) is listed second.
metal;nonmetal
Metals are to the ____ of the periodic table and nonmetals are to the _______ of the periodic table.
Left, right
An ionic bond forms between a ___ and _____. A covalent bond forms between a _____ and ____.
metal / nonmetal
nonmetal/nonmetal
Explain why nitrogen and oxygen cannot form an ionic bond.
They are both nonmetals and would form a covalent bond.
Why is this the incorrect name for the compound shown below:
Al2O3 = aluminum oxygen
Endings should always be "ide".
aluminum oxide
What is the correct formula for dihydrogen monoxide?
H2O
Is the following an ionic or covalent compound?
calcium chloride
ionic
Which atoms would most likely form a bond by sharing electrons?
H and O
Na and Cl
Ca and K
H and O- these are both nonmetals
Explain why sulfur and bromine cannot form an ionic bond?
Both atoms are nonmetals and need to take electrons to obtain a full outer shell.
Name this compound:
CO2
Carbon dioxide
What is the correct chemical formula for magnesium phosphide?
Mg3P2
What is the "anion" in the compound shown below?
Magnesium bromide
Bromine (this is a nonmetal). Nonmetals take electrons and become anions (negative).
Which atoms would most likely form a bond by transferring electrons?
P and S
Ca and Cl
I and S
Ca and Cl- these atoms will form an ionic bond.
During an ionic bond, calcium will (give/take) electrons and nitrogen will (give/take) electrons.
give; take
Name these compounds.
CaI2
K3N
Calcium iodide
Potassium nitride
A student states the chemical formula for strontium chloride is SrCl. Why is this incorrect?
The correct chemical formula should be SrCl2.
3
How is the octet rule related to chemical bonding?
Most atoms bond to form an octet.
Draw the Lewis dot structures for calcium (Ca) and chlorine (Cl). Then use arrows to show the flow of electrons when these atoms form an ionic bond.
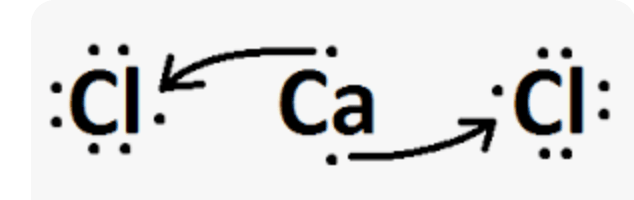
Name these three compounds:
K2O
CCl4
MgF2
potassium oxide
carbon tetrachloride
magnesium fluoride
Which formulas is not correct?
lithium sulfide = Li2S
beryllium bromide = BeBr2
aluminum oxide = Al3O2
aluminum oxide = Al2O3
What is group 14's charge?
+/- 4