Two or more different atoms chemically combined.
What is Compound?
A molecule made up of two or more atoms of the same type of element.
What is Pure Element / Elementary Substance?
If electronegativity difference between atoms is equal to or greater than 2.0, they will form this type of chemical bond.
Electronegativity difference of 0.45 would result in this type of chemical bond forming.
What is Non-Polar Covalent?
Answer both:
- PE or Compound
- Covalent or Ionic
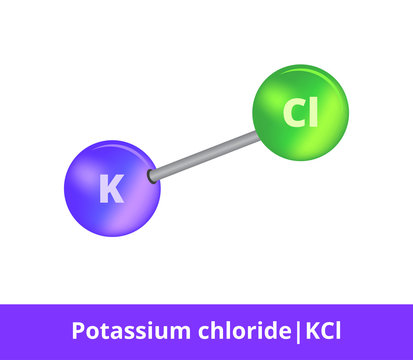
What is Ionic Compound?
A positively charged ion.
What is Cation?
Sodium Chloride (NaCl) is an example of this type of molecule?
What is Compound?
Chemical bond in which valence electrons are joined in electron pairs.
What is Covalent Bond?
In terms of electron pairs, this is the type of chemical bonds this molecule will have.
What is Single Covalent Bond?
Answer both:
- PE or Compound
- Polar Covalent or Non-Polar Covalent
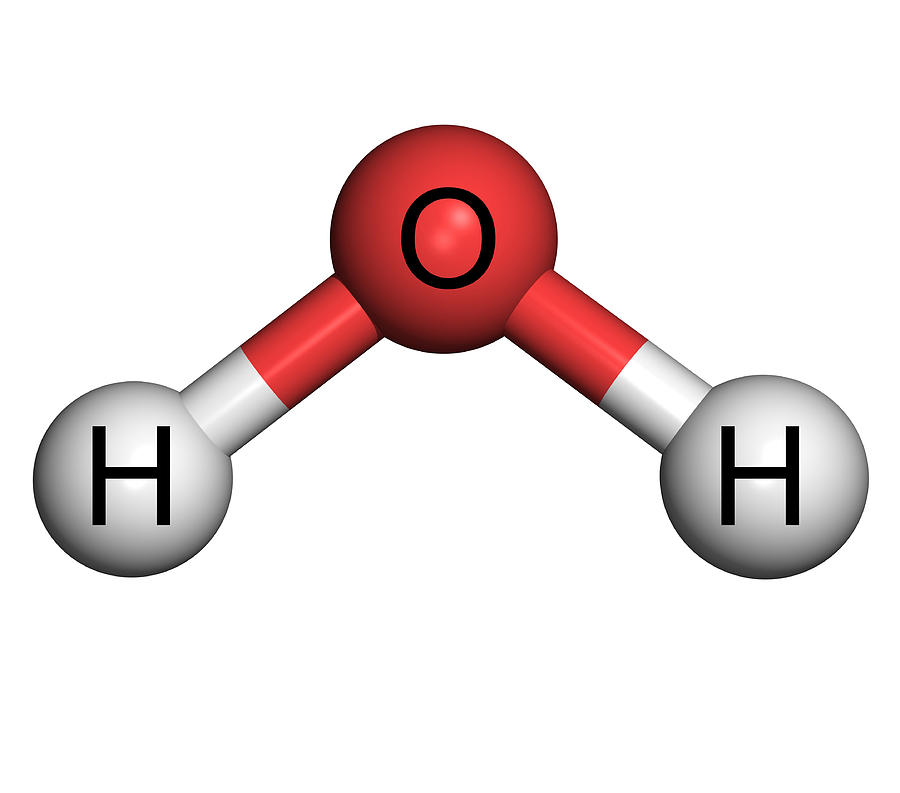
What is Polar Covalent Compound?
When electrons from different atoms are grouped together in a chemical bond.
What is Electron Pair?
Octasulfur is an example of which type of molecule?
What is Pure Element?
Chemical bond in which electron(s) from a metal are stripped and added to a nonmetal.
What is Ionic bond?
The sticking together of an anion and a cation due to their electrostatic attraction refers to this.
What is Ionic Bond?
Type of chemical bond between Nitrogen and Oxygen.

What is Polar Covalent?
An atom's ability to attract the electrons of another atom for chemical bonding.
What is Electronegativity?
Hexoxide (O6) is an example of this type of molecule.
What is Pure Element?
A covalent bond between two atoms, with 4 electrons being shared across one single bond would be this type of covalent bond.
What is Double Covalent Bond?
If a cation from group 2A formed a neutral ionic bond with only one anion atom, it would have to be from this group.
What is Group 6A?
Type of chemical bond between Chlorine and Lithium.

What is Ionic?
This type of chemical bond can be both a pure element and a compound.
What is Non-Polar Covalent Bond?
This is the reason atoms form chemical bonds.
What is...
1) Fill octet
2) Move to lowest PE possible
The satisfactory point of minimal energy between two atoms in a chemical bond.
What is Bond Length?
Type of chemical bond between Sulfur and Phosphorus.

What is Non-Polar?