The intramolecular attraction happening when the electronegativity difference is 1.9
What is ionic bond?
The difference between intra and intermolecular attractions
What is
intra is between atoms and
inter is between molecules?
Two properties Magnesium has based on its intermolecular forces
What is conductive and malleable?
(or ductile or durable or high boiling point)
The geometry of CO2
What is linear?
Draw the Lewis structure for H2S.
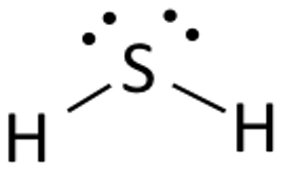
The intramolecular force occuring between hydrogen and oxygen
What is polar covalent bond?
The three different types of intermolecular attractions
What is london, dipole, and hydrogen bonding?
Two properties of K-F based on its intermolecular properties
What is crystalline lattice and conductive dissolved in water? (or rigid solid or a brittle salt or nonconductive solid or very high meliting or very high boiling point)
The geometry of NO3
What is trigonal planar?
Draw the Lewis structure for PCl5.
The intramolecular force at play when hydrogen bonding is occurring
What is polar covalent bonding?
The strongest intermolecular force happening in carbon tetrachloride
What are london forces or dispersion forces?
(or van der waals forces)
 Two properties of ammonia, based on its intermolecular forces
Two properties of ammonia, based on its intermolecular forces
What is soluble in water and attractive to charge? (or liquid at room temperature or low viscosity or medium boiling point)
The geometry of SO2
What is bent?
Draw the Lewis structure for GeO2 and tell how many electron domains it has.
What is 2?

The intramolecular force happening between Lithium and Fluorine
What is ionic bonding?
The strongest intermolecular force occurring in hydrogen sulfide
What is london forces or dispersion forces or van der waals forces?
Two properties of plastics (polymers) which are made of very long chain hydrocarbons, due to their intermolecular forces
What are nonconductive and durable?
(or flexible or do not dissolve in water or lowing melting point for their mass)
The geometry of AsF5
What is trigonal bipyramidal?
Draw the Lewis structure for SbCl3 and tell how many bonds and lone pairs there are in it.
What is 3 bonds and 1 lone pair?

The intramolecular force happening in sulfur dioxide
What is polar covalent bonding?
All the intermolecular forces in ammonia
What are london, dipole, and hydrogen?
Two properties that molecules with hydrogen bonds have
What are high melting and boiling points for their mass?
(or good surface tension or soluble in polar liquids)
The geometry of SbH3
What is trigonal pyramidal?
Draw a Lewis Structure for BF3 and tell its number of bonds and lone pairs and its geometry.
What is 3 bonds, 0 lone pairs, trigonal planer?
