This type of bond exists most often between two nonmetals, resulting in the sharing of electrons.
What is covalent bonding?
A molecule of chlorine gas (Cl2) would be described as this shape. 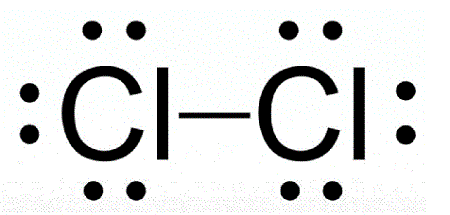
What is linear?
The Lewis structure of the compound CH4 would be drawn as this:
What is:
Molecules with a symmetrical distribution of charge are described by this polarity.
What is nonpolar?
This athletic wear company sports the following logo.
What is Nike?
This type of bonding results in the transfer of electrons between a metal and a nonmetal.
What is ionic bonding?
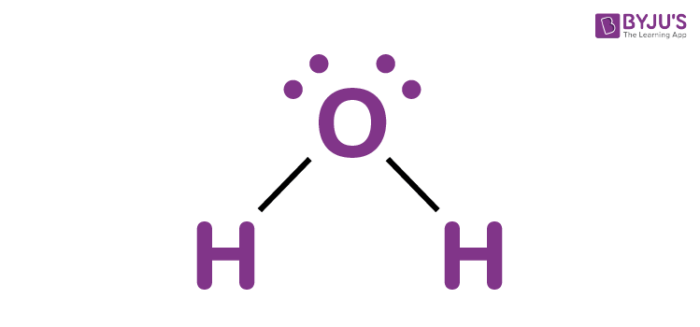 Lewis structures of water (H2O) must be drawn with this shape.
Lewis structures of water (H2O) must be drawn with this shape.
What is bent?
In the Lewis structure of an ionic compound, this type of ion must be drawn with a full octet of electrons, including brackets.
What is the anion (negative, nonmetal ion)?
The polarity arrow: for an HF bond would be added in this direction.
for an HF bond would be added in this direction.
What is: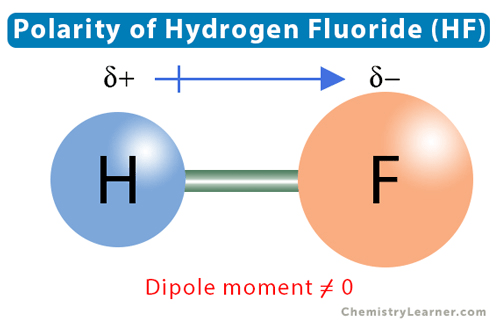
This Mexican fast food chain boasts the slogan, "Live Mas." 
What is Taco Bell?
A triple covalent bond, such as the one shown, results in the sharing of this number of electrons.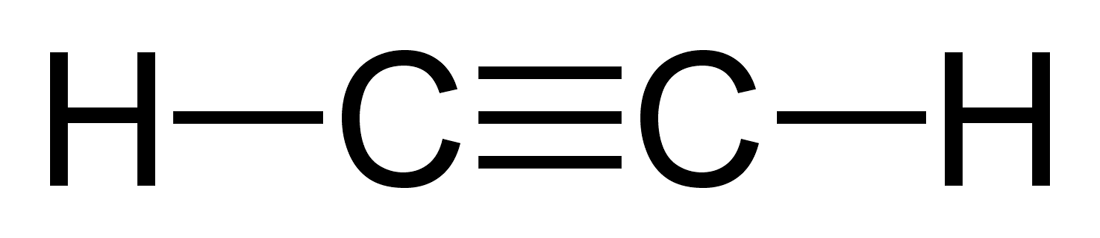
What is 6 electrons (3 pairs)?
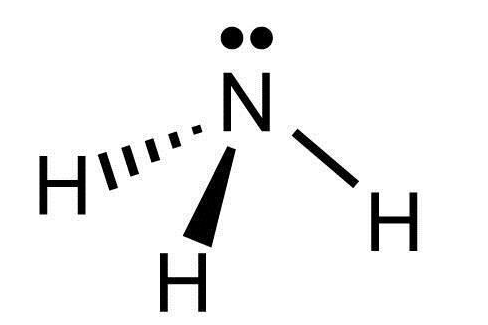 Ammonia's (NH3) molecular shape would best be identified as this.
Ammonia's (NH3) molecular shape would best be identified as this.
What is trigonal pyramidal?
The Lewis structures of water molecules (H2O) must be drawn with this general shape.
(Bonus points for drawing structure)
What is bent? 
A nonpolar covalent bond is described as having an electronegativity difference less than or equal to this value.
What is 0.4?
This food manufacturing giant makes everything from Cheerios cereal and Kit-Kats, to Purina dog food.
Who is Nestle?
This type of bond is characterized by an electronegativity difference between 0.5 and 1.7.
What is a polar covalent bond?
Methane molecules have four carbon-hydrogen bonds, forming this shape. 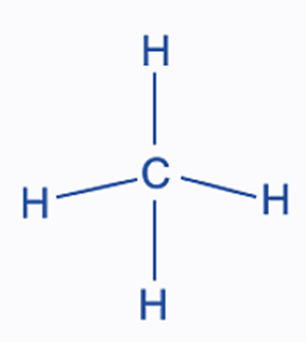
What is tetrahedral?
The Lewis structure for carbon dioxide (CO2) must be drawn as this.
What is: 
What is hydrogen bonding?
"What would you do?" for a chocolate covered ice cream bar from this company?
What is Klondike?
This type of chemical bond is described as a "sea of mobile electrons" loosing holding together a lattice of nuclei.
What is metallic bonding?
Molecules of H2S would best be described as this shape.
What is bent?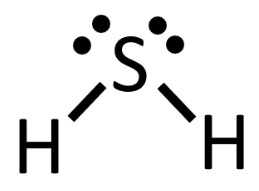
The Lewis structure for the compound formed between phosphorus (P) and nitrogen (N) and would be drawn as this.
What is: 
The sodium ions in a salt water solutions would be attracted to this atom in the water molecules.
What is oxygen?
This airline is one of the largest in the world, employing over 100,000 people worldwide.
What is Lufthansa?