This bond results from the unequal sharing of electrons
Polar covalent bond
CO2
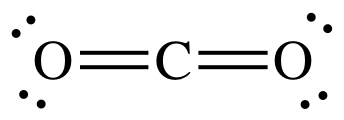
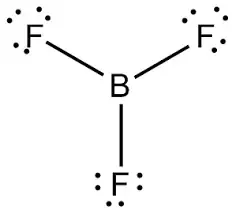
Draw BF3
Shape and bond angle for H2O
Bent and 109.5
Is the following molecule Polar or nonpolar, and what is the main intermolecular force: Cl2
Nonpolar and LDF
This is the weakest of all types of bonds
London dispersion forces
Ba3N2
[Ba]+2 [N]-3 [Ba]+2 [N]-3 [Ba]+2
Draw CF2Cl2

Shape and bond angle for SO3
Trigonal planar and 120
Is the following molecule polar or nonpolar and what is the main intermolecular force: H2O
Polar and Hydrogen Bonding
Ionic bonds have an electronegativity of
Greater than 1.7
NO+1
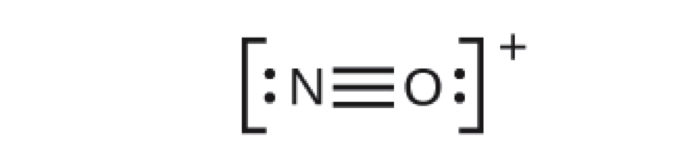
Draw SF6

Shape and Bond angle for CH4
Tetrahedral and 109.5
Is the following molecule polar or nonpolar, and what is the main intermolecular force: CO2
Nonpolar and LDF
Draw the dipole arrow and the partial charges for the following bond: H-Cl

NO3-1

Draw CO3-2

Shape and bond angle for NH3
Trigonal pyramidal and 109.5
Is the following molecule polar or nonpolar and what is the main intermolecular force: SO2
Polar and Dipole-Dipole
Rank the following bonds from strongest to weakest: London forces, dipole-dipole, polar covalent, hydrogen
Polar covalent, hydrogen, dipole-dipole, london
C3H6O

Draw Cl3PO
Shape and bond angle for PCl5
Trigonal bipyramidal and 90, 120 and 180
Is the following molecule polar or nonpolar and what is the main intermolecular force: NH3
Polar and Hydrogen Bonding