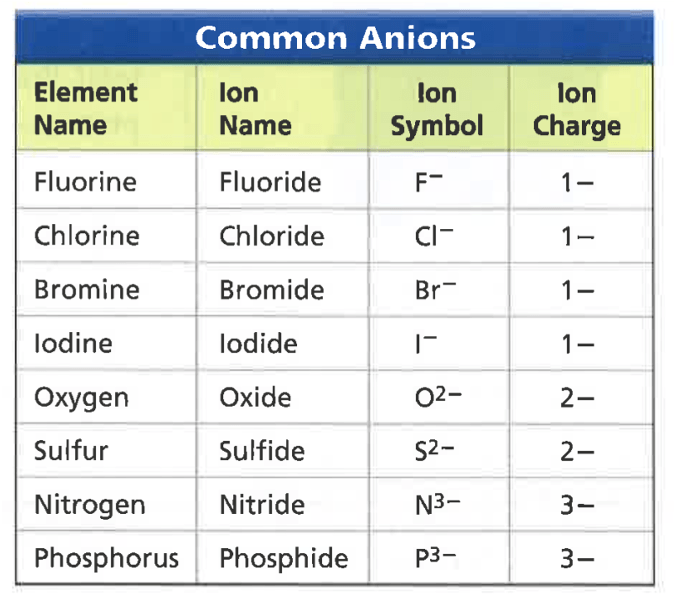
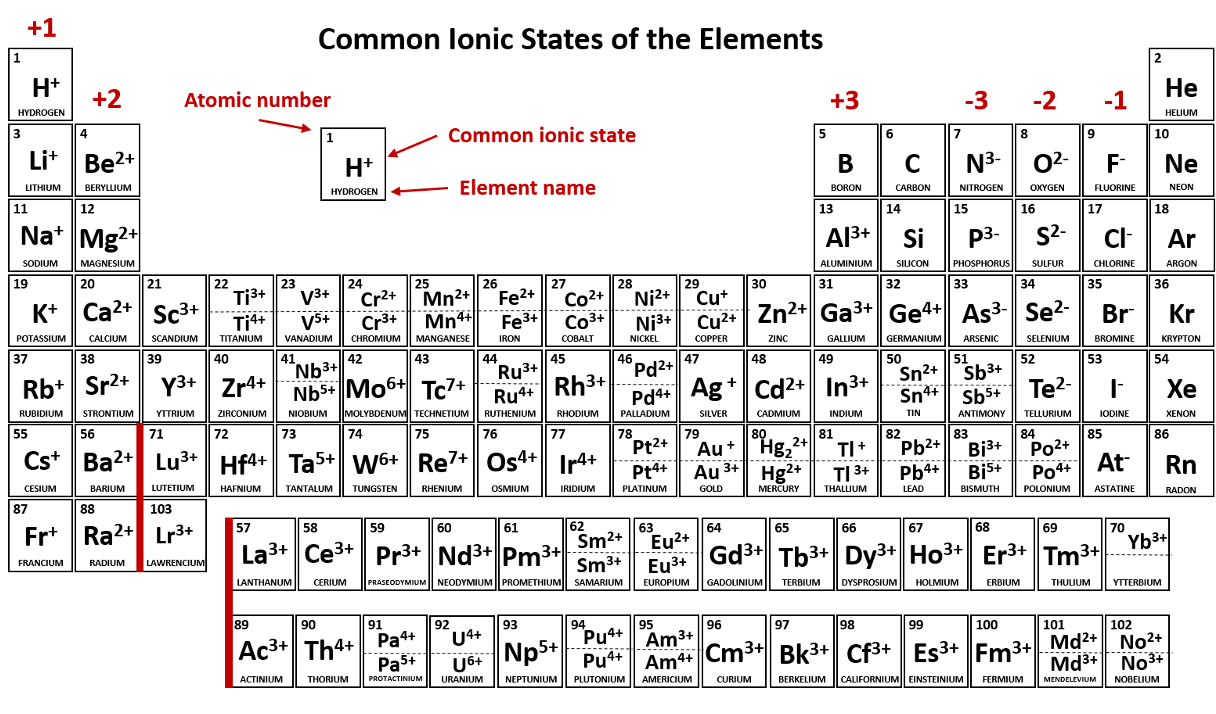
(Ionic Compounds)
(Covalent Compounds)
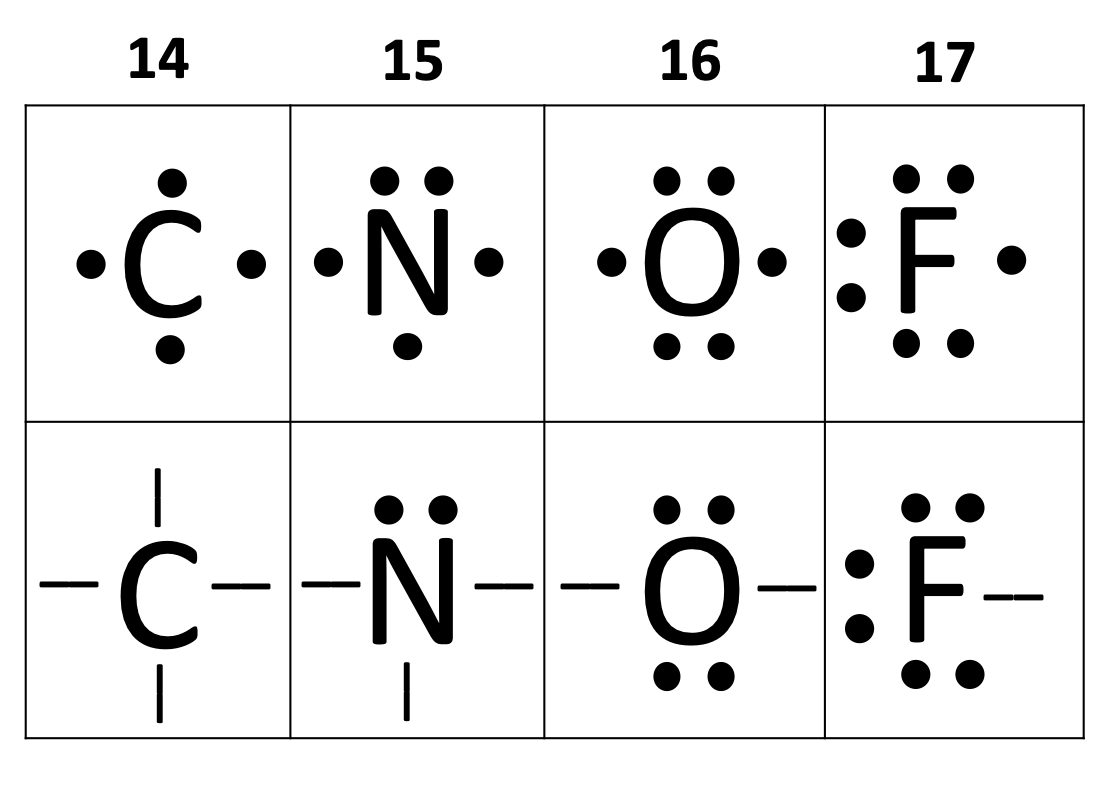
How many valence electrons does carbon have if it is in Group 14?
4
four
An ionic bond will always form when these two types of elements react.
metal and a nonmetal
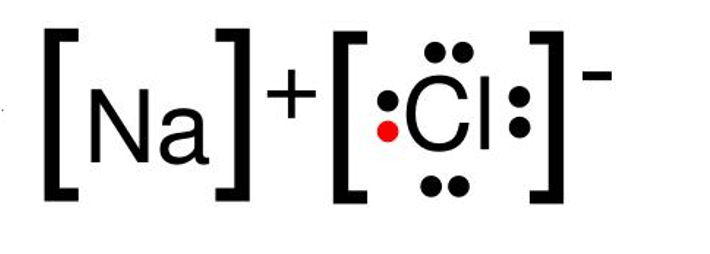
A covalent bond will always form when these two types of elements react.
nonmetal and nonmetal
What is the name of KBr
potassium bromide
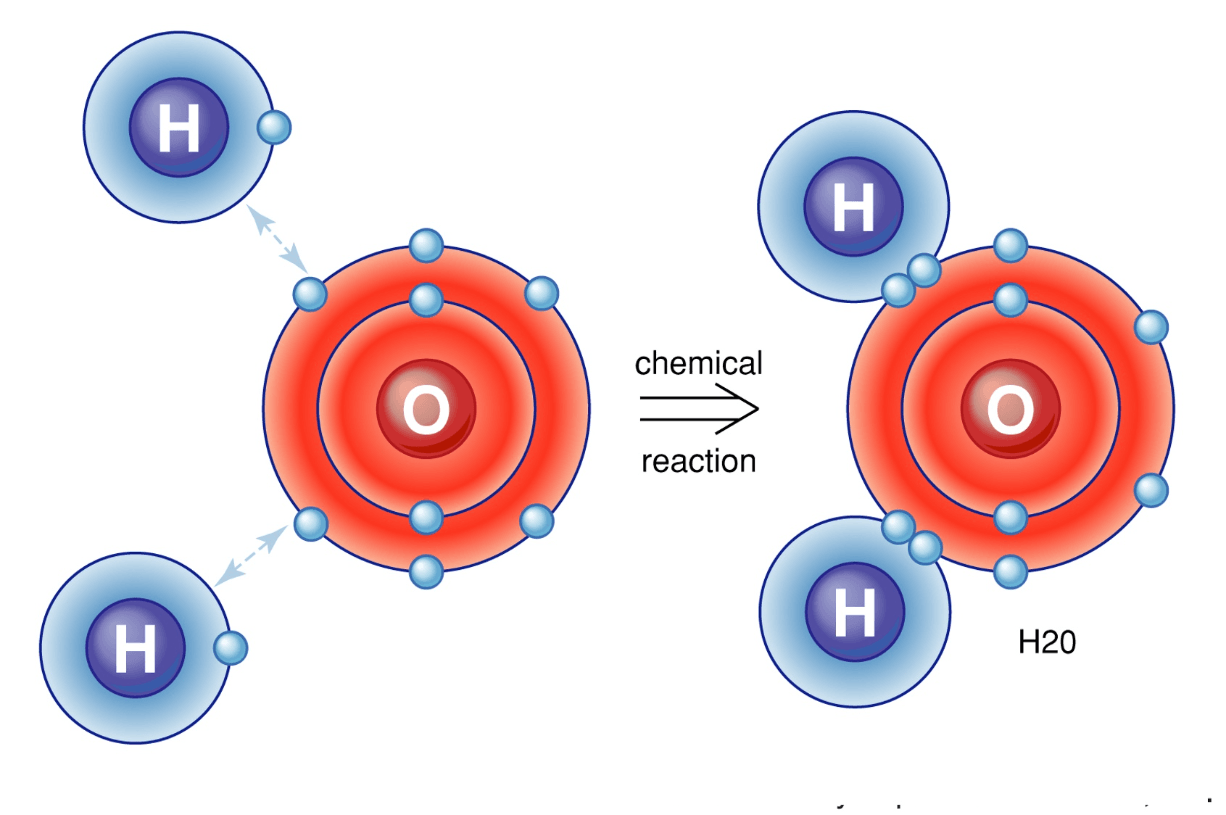
What is the name of H2O
dihydrogen monoxide (NOT monooxide)
Which electrons are responsible for the reactivities of the elements?
valence electrons
outermost electrons
electrons in outermost shell
An atom or molecule that has a net negative or positive charge is called a/an _____.
ion
When an atom hogs/attracts electrons in a covalent bond, it is said to be _____.
What is the formula for lithium hydride
LiH
What is the formula for phosphorus trihydride
PH3
Elements require how many electrons in the outermost shell to be considered stable?
8
eight
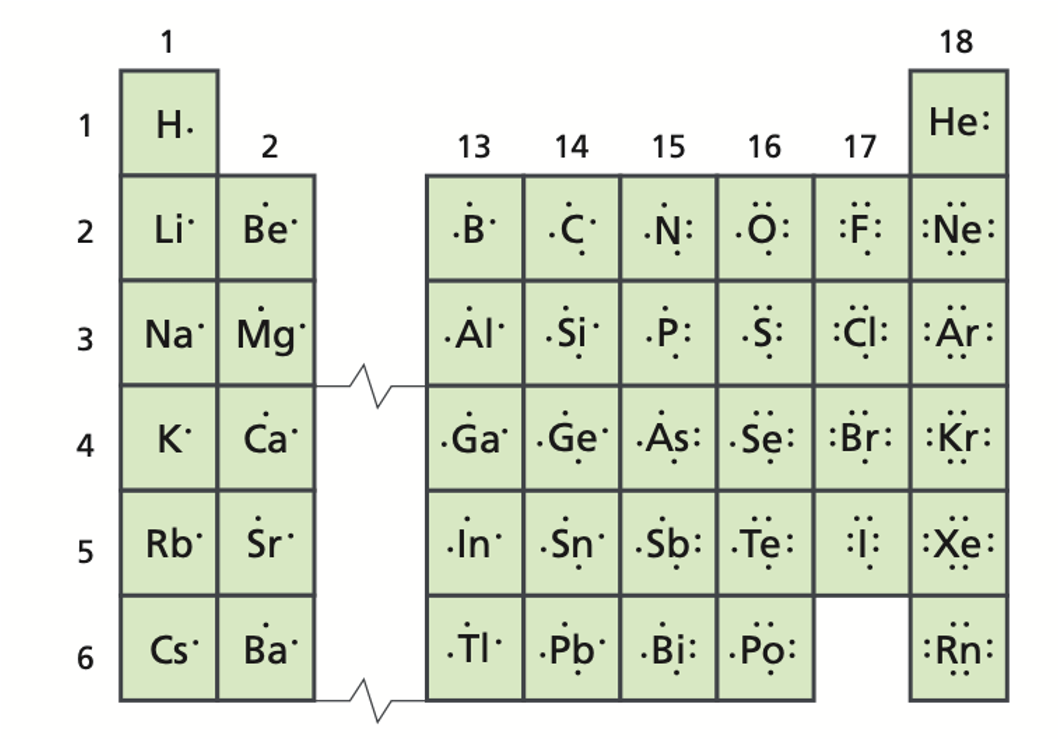
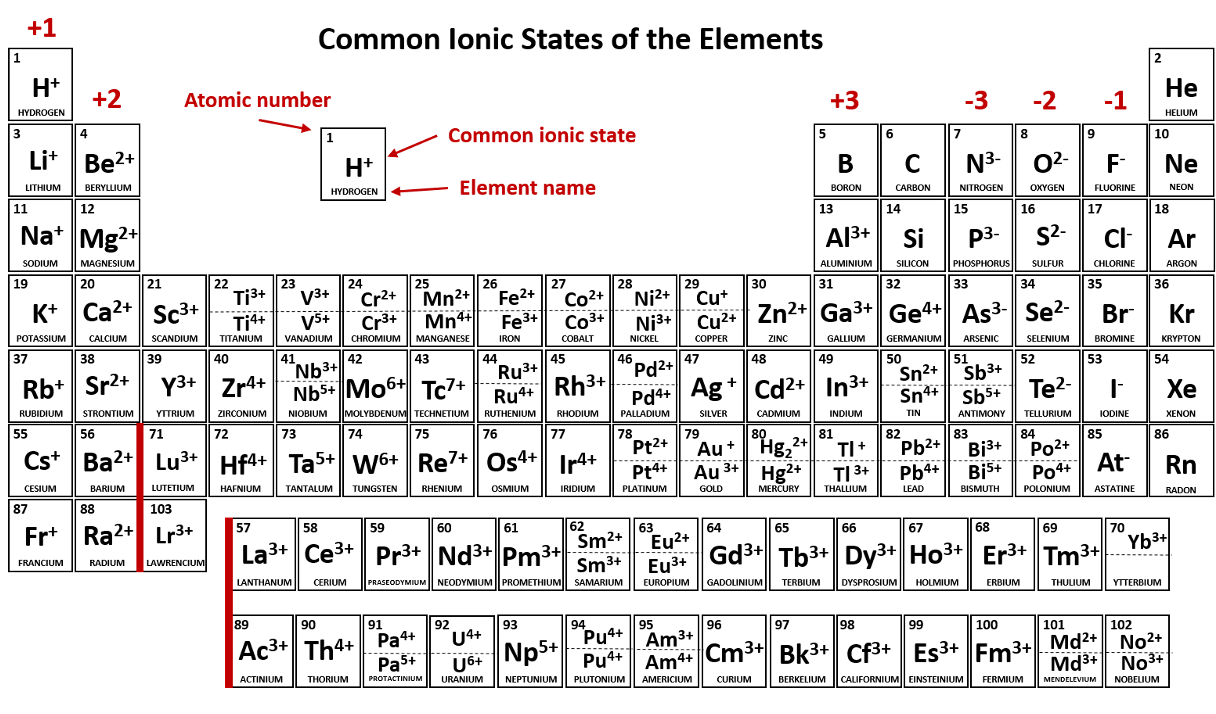
A positively charged ion is called a/an _____.
cation (Li+, Na+, Cu+, Cu2+, NH4+)
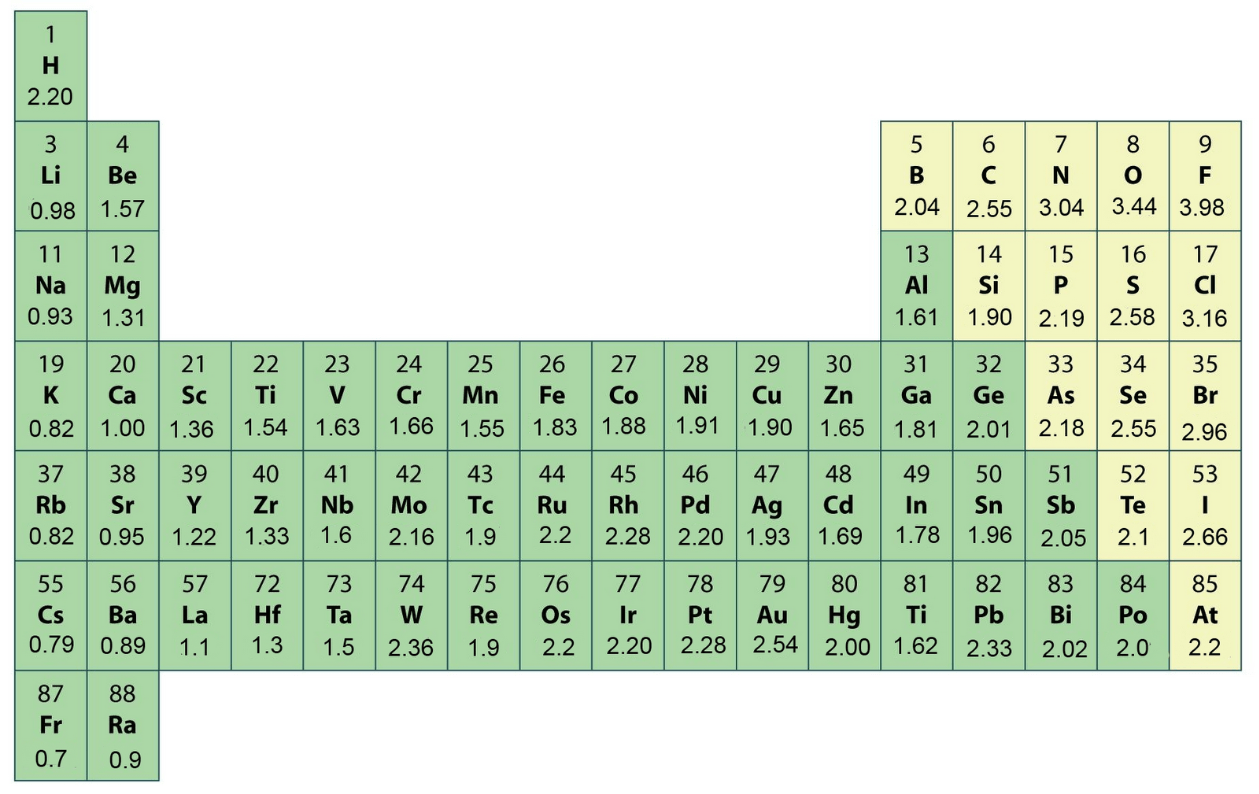
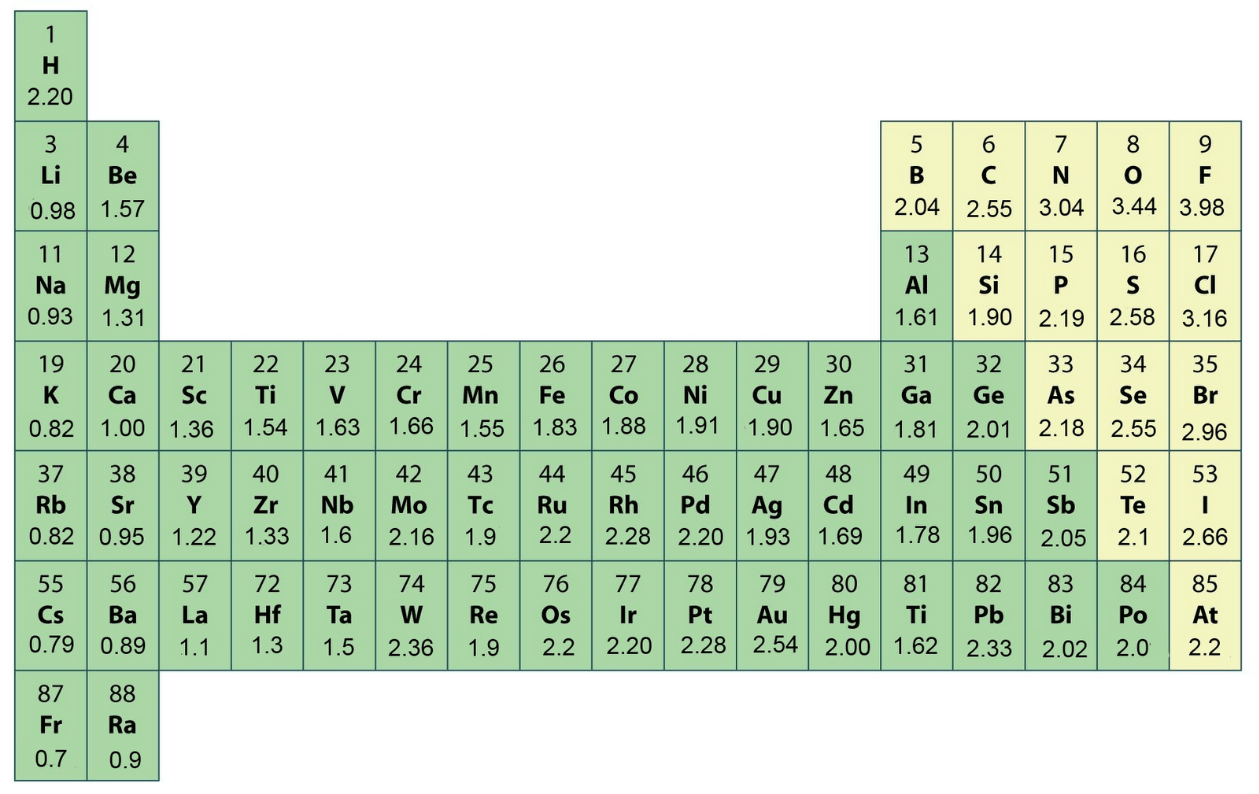
Which atom is the LEAST electronegative atom?
Francium (Fr)
What is the name of Na2SO4?
sodium sulfate
What is the name of CO?
carbon monoxide (NOT monooxide)
When two or more atoms share electrons.
covalent bond
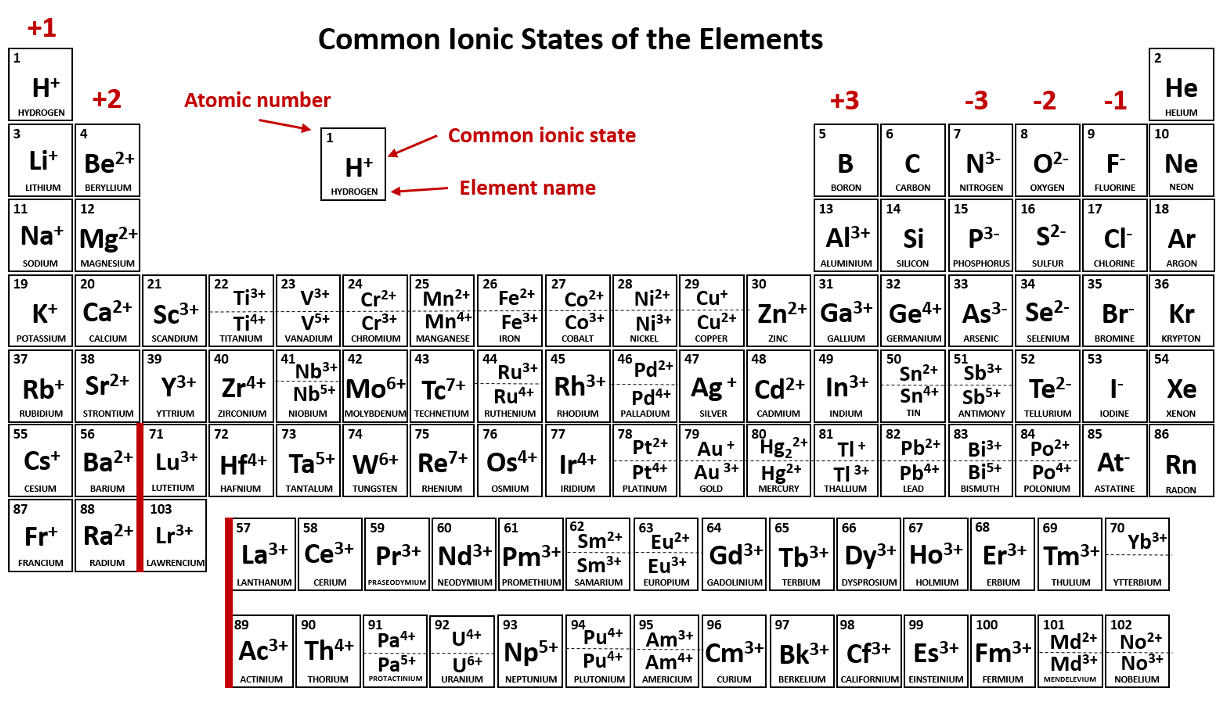
Group 1 metals will always ionize to form ions with a ____ charge. (Give number and sign; e.g. 1-, 1+, 2+, etc.)
1+

What type(s) of bonds are shown in the compound below?
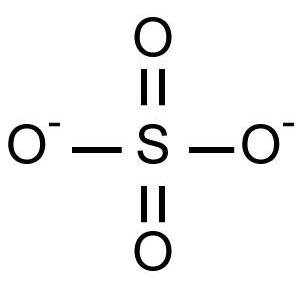
2 single bonds and 2 double bonds
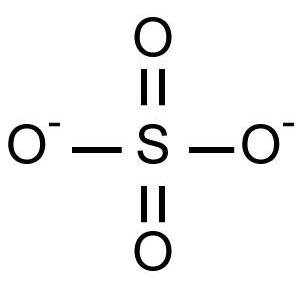
What is the formula for potassium carbonate?
K2CO3
What is the name of N2O4?
dinitrogen tetroxide (NOT tetraoxide)
If an atom loses an electron, then that atom will become ____ charged.
positively charged
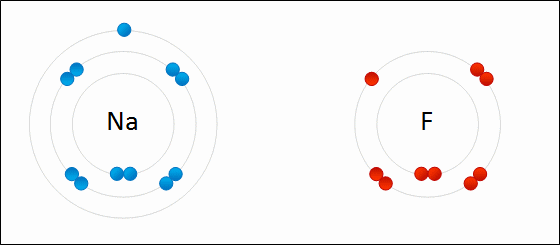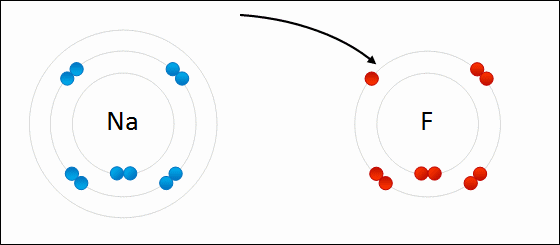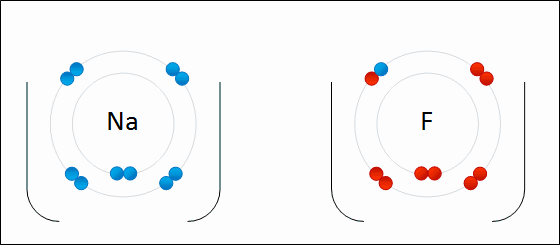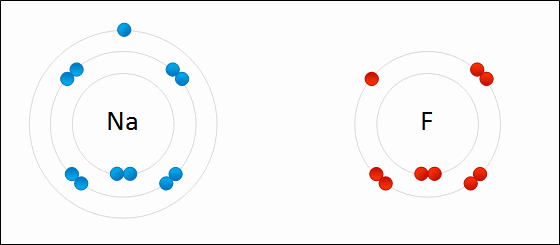
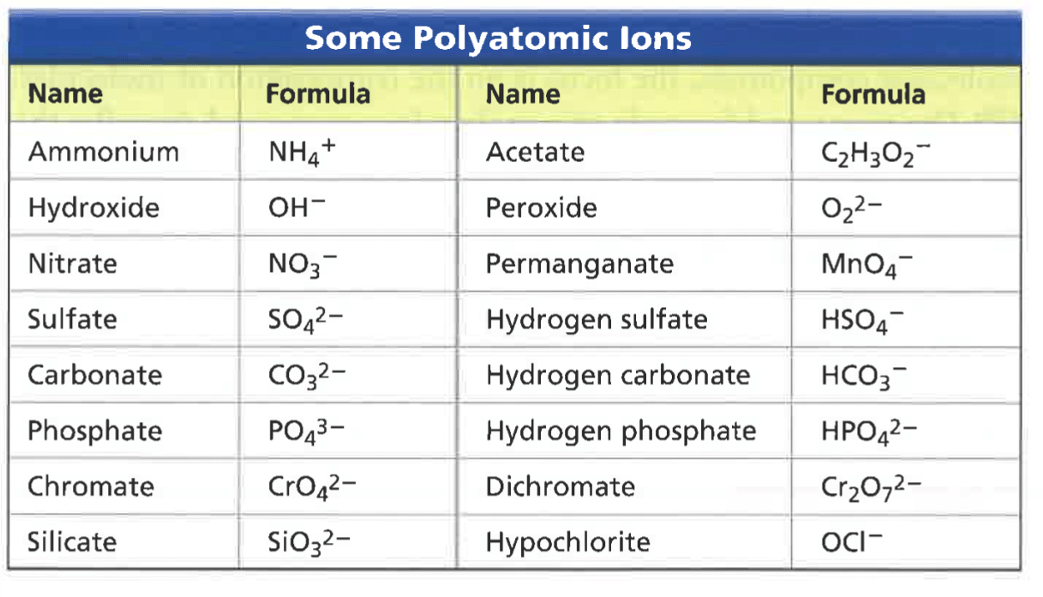
Ions that that contain multiple atoms/elements are called ____.
polyatomic ions
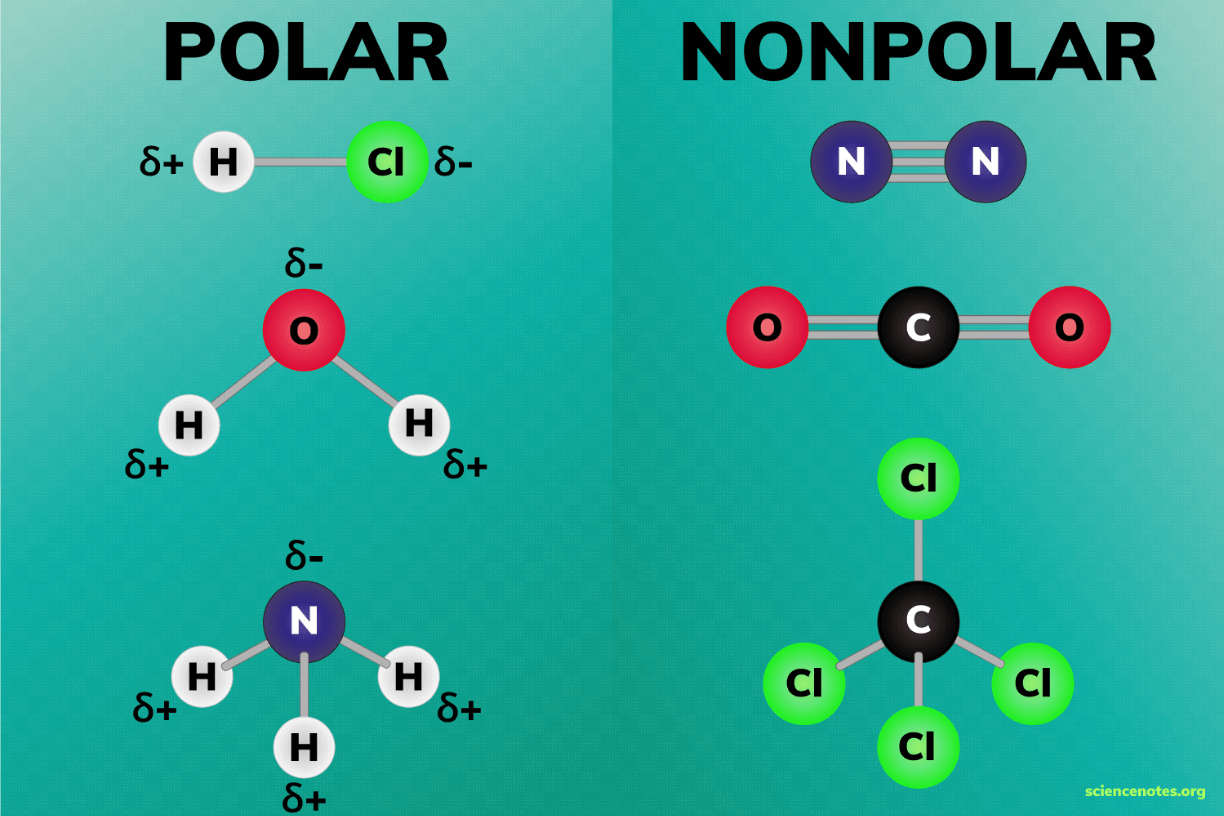
Molecules that have an uneven distribution of electrons are called _____.
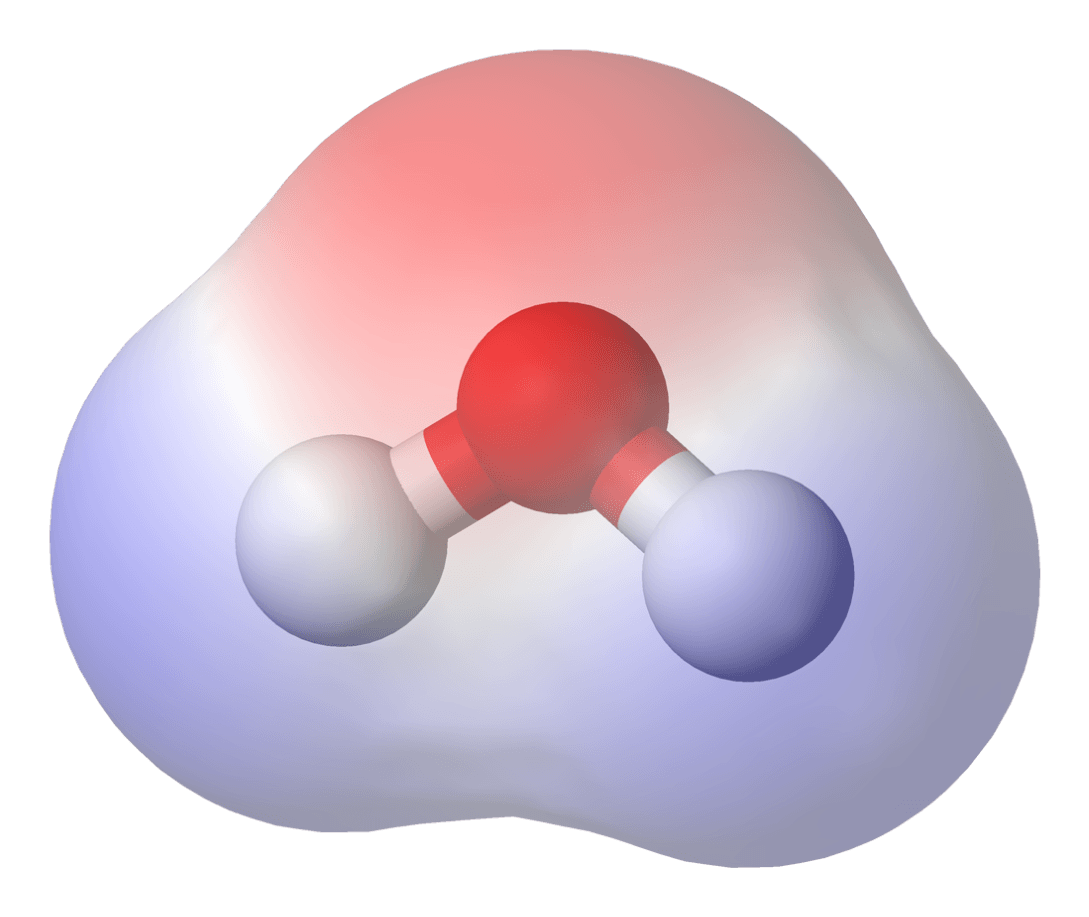
polar
What is the name of CuO?
copper(II) oxide
What is the formula for tetracarbon octahydride?
C4H8
The bond formed when two or more atoms transfer electrons.
ionic bonding
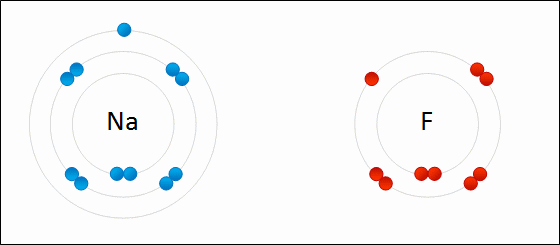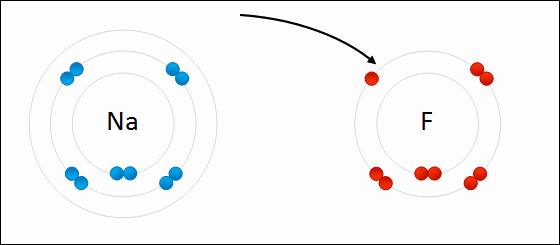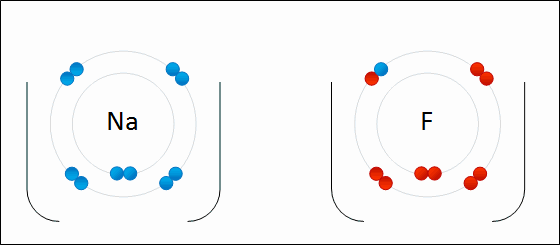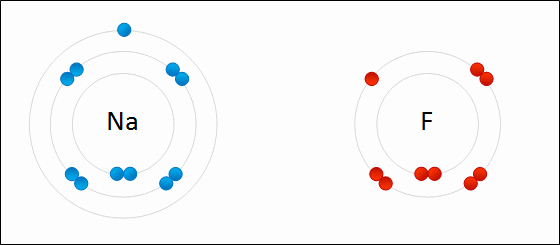
A negatively charged ion is called a/an _____.
anion
How many electrons are in a covalent bond?
two

What is the formula for sodium iodide?
NI
What is the formula for carbon tetrachloride?
CCl4
If an atom gains an electron, then that atom will become ____ charged.
negatively charged

Metals in Group 2 always form ions that have a ____ charge. (Give number and sign; e.g. 1-, 1+, 2+, etc.)
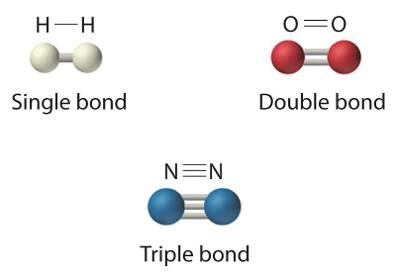
Which type of bond is the strongest?
triple bond
What is the name of NH4F?
ammonium fluoride
What is the name of NO?
nitrogen monoxide (NOT monooxide)
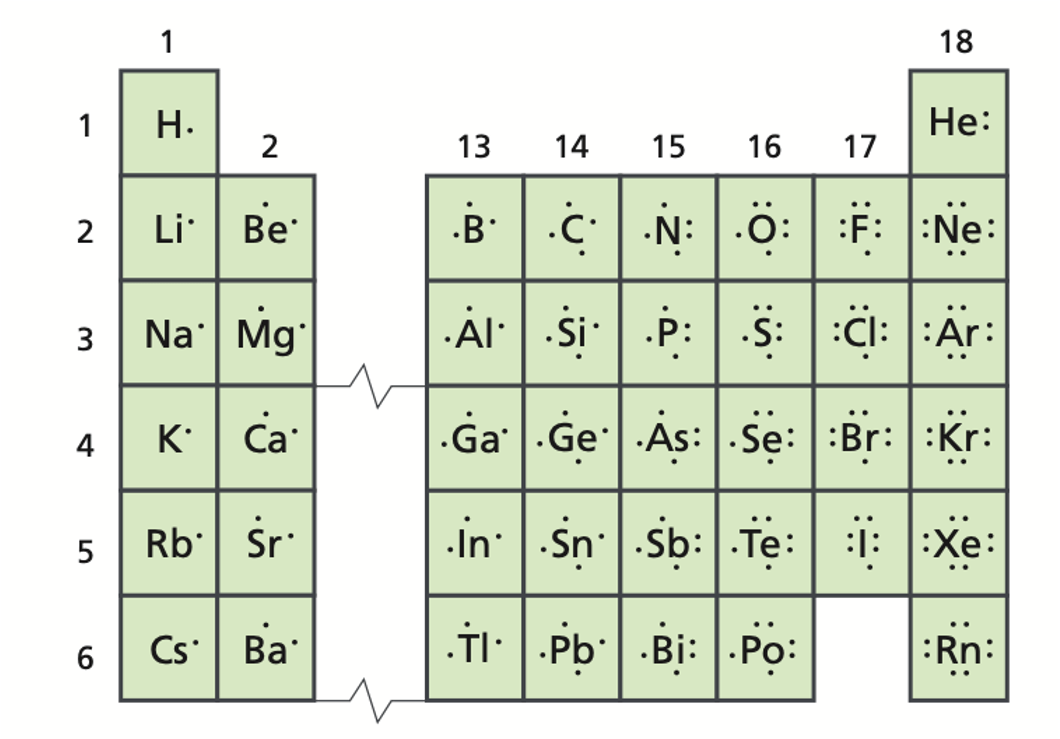
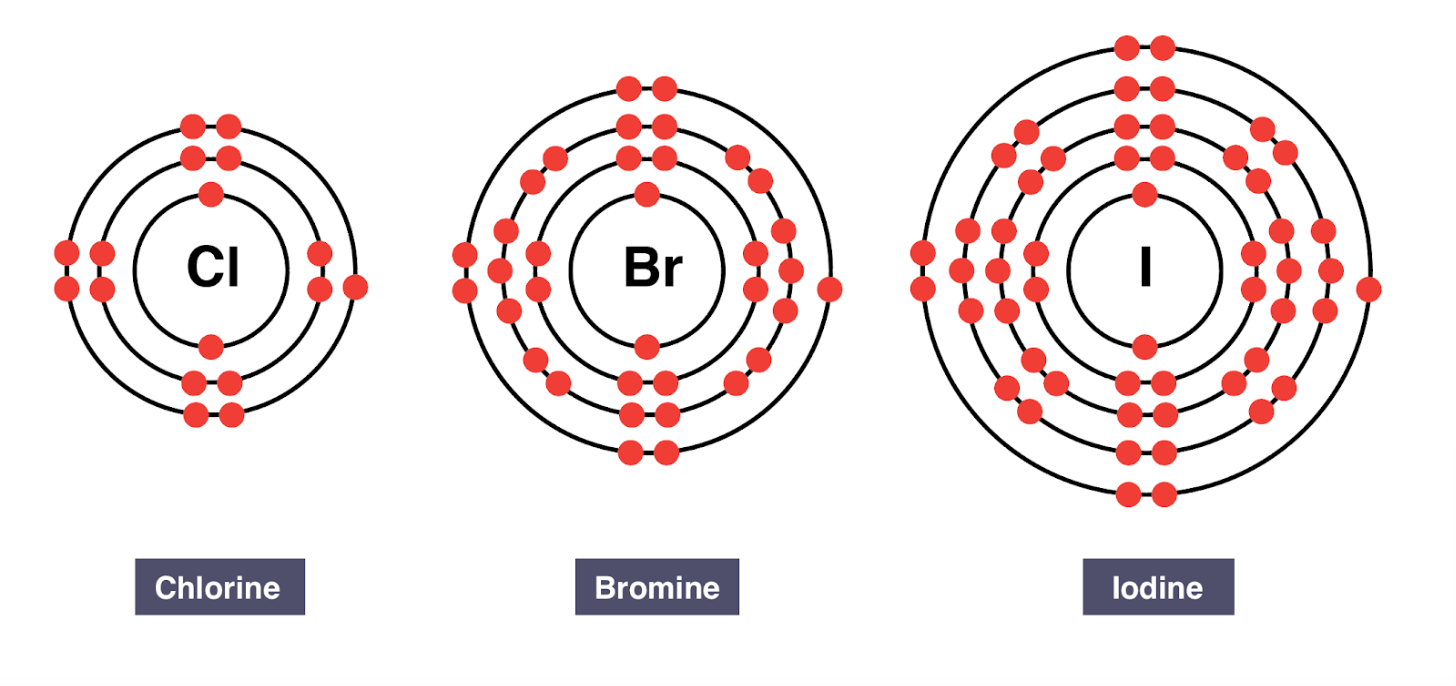
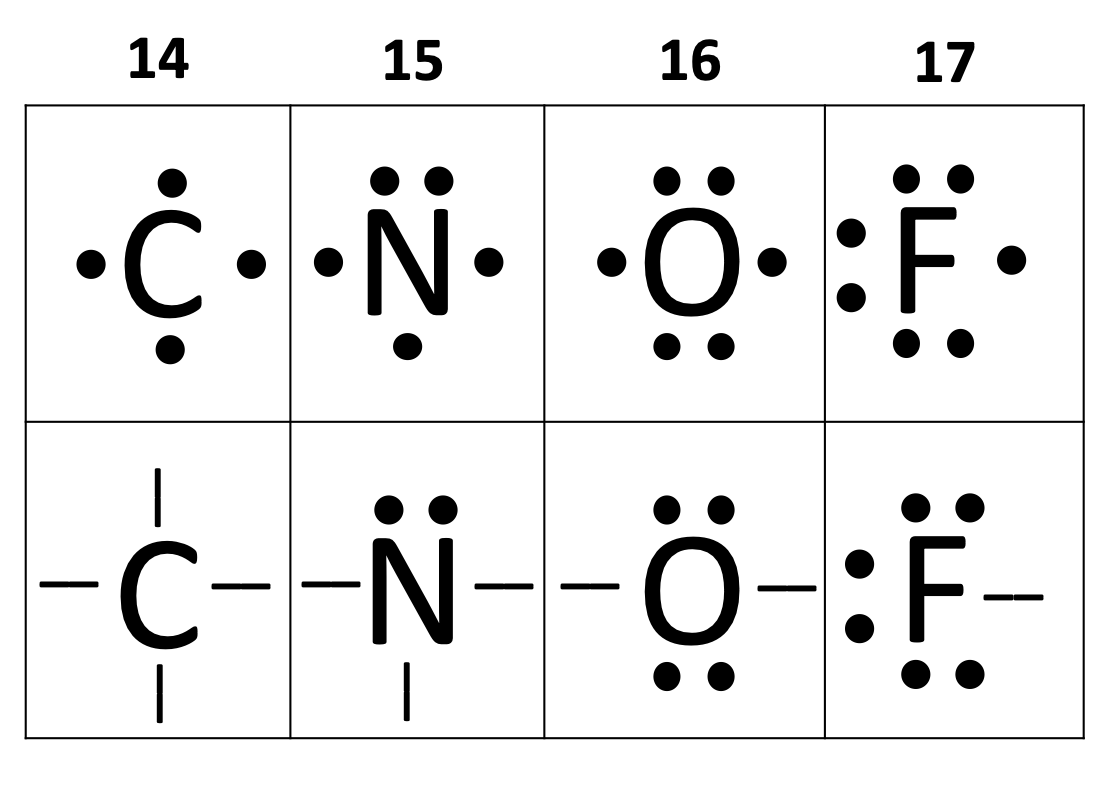
The following diagrams only show the valence electrons for each element/atom and are called _______.
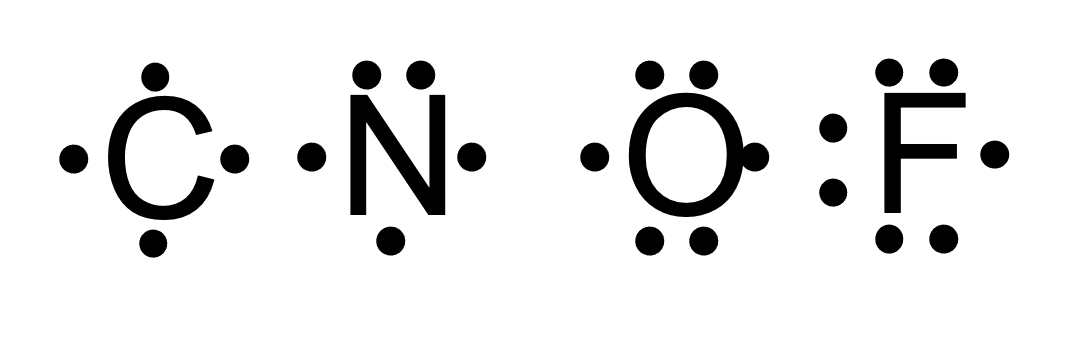
electron dot diagrams
Halogens always ionize to form ions with a ____ charge. (Give number and sign; e.g. 1-, 1+, 2+, etc.)

Which element is the MOST electronegative?
Fluorine
What is the name of Mg(NO3)?
magnesium nitrate
What is the name of H2O2?
dihydrogen dioxide
How many electrons does sulfur need to have a full valence shell if it is in Group 16?
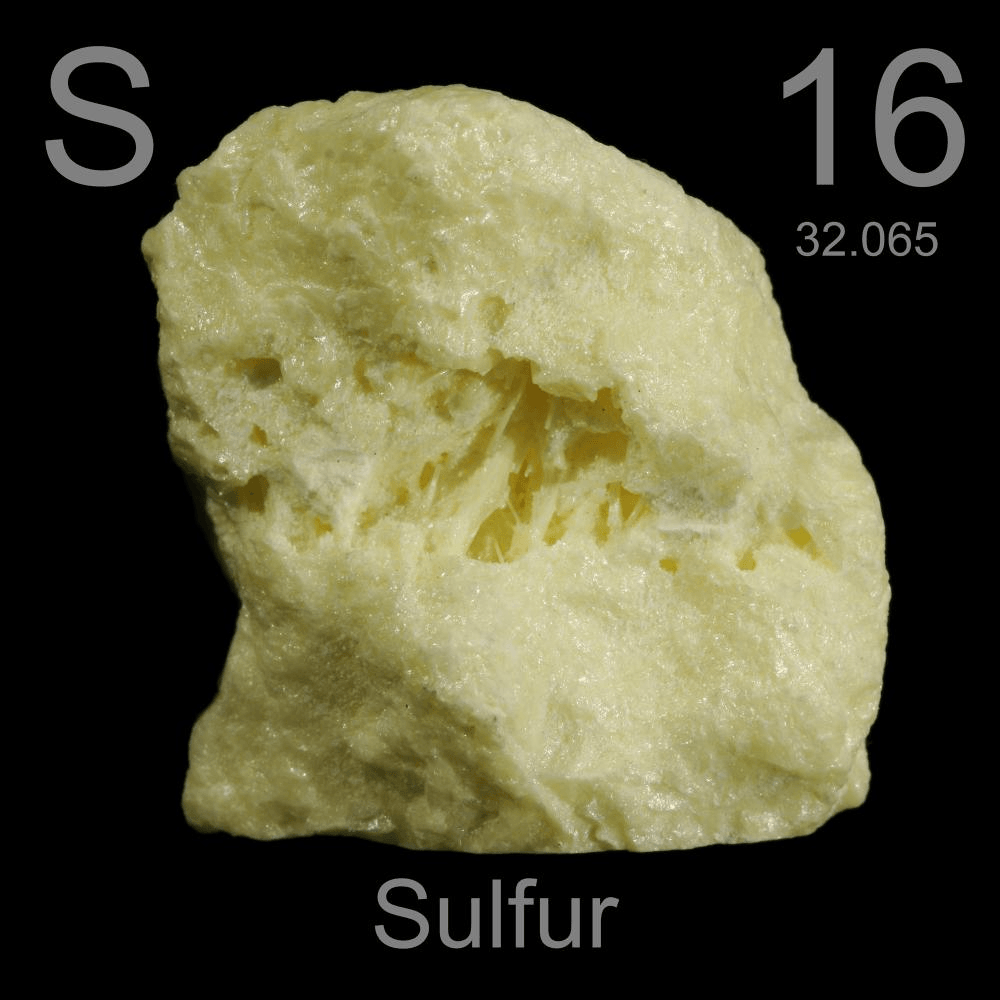
2
two
What charge does copper have in the ionic compound CuCl2?
copper(II)
How many bonds can carbon form? (i.e. How many valence electrons does carbon need)

Figure: charcoal is mostly pure carbon
4
four
What is the formula for iron(II) chloride
FeCl2
What is the formula for tetraboron tetrahydride?
B4H4
Ionic compounds can form larger, ordered 3D structures called ___
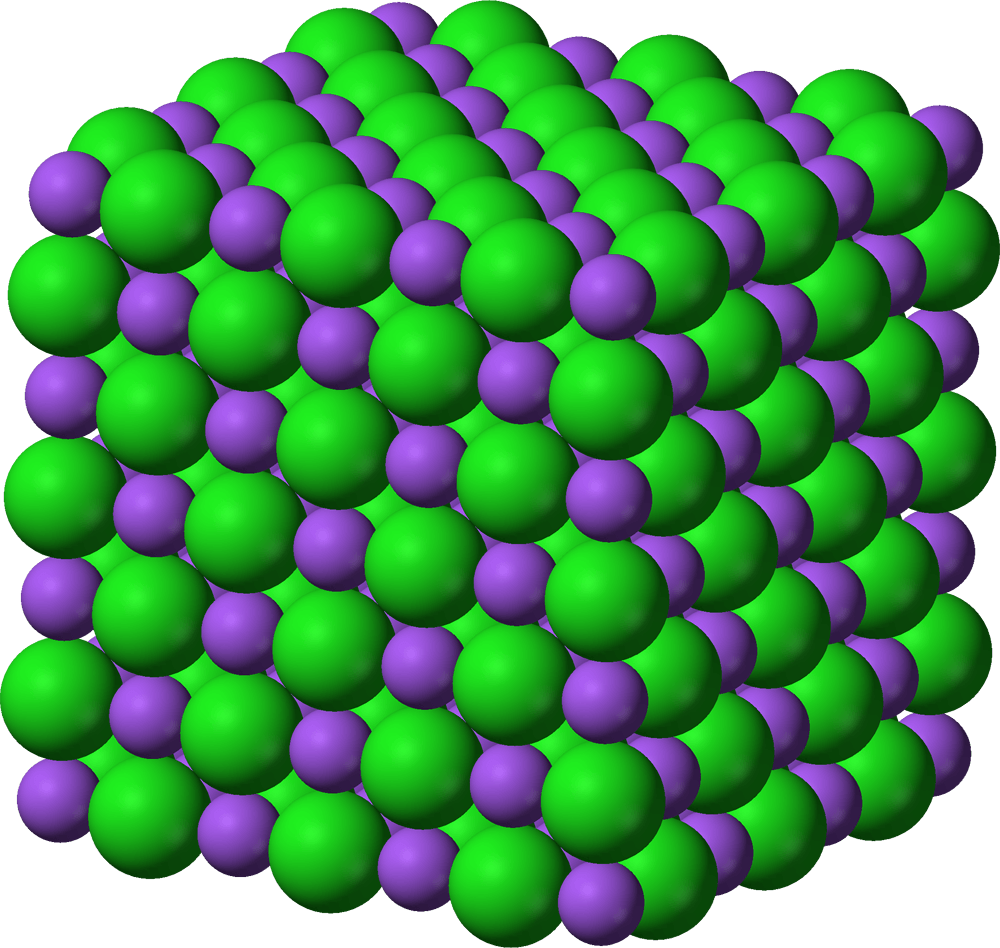
crystals/crystal lattices
What is the charge on iron (Fe) in the ionic compound Fe2O3?
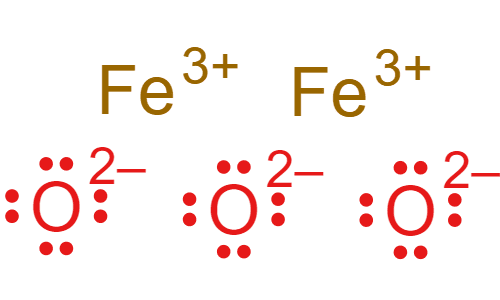
How many bonds can nitrogen form? (i.e. How many valence electrons does carbon need)

What is the formula for ammonium sulfate?
(NH4)2SO4
What is the formula for pentasulfur hexanitride
S5N6