Why do elements form bonds?
To have full valence shells to be stable
A positively charged ion is called a/an _____.
cation
Which element is the MOST electronegative?
Fluorine
Does CO₂ contain ionic or covalent bonds?
covalent
Which group of non-metals does not tend to form bonds due to their full valence shell?
Noble gases
This bond is when two or more atoms share electrons.
covalent bond
A negatively charged ion is called a/an _____.
anion
True or False: ALL elements want eight valence electrons.
False. (ex: hydrogen and helium need only 2, sulfur and phosphorous can hold 10, boron is happy with 6)
Does HF contain covalent or ionic bonds?
covalent
What is the name of MgCl₂?
Magnesium chloride
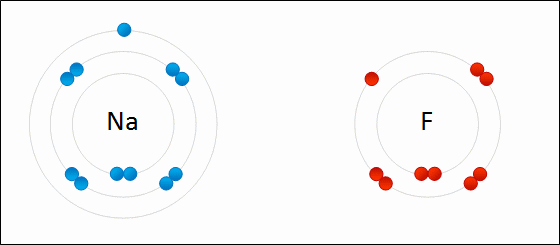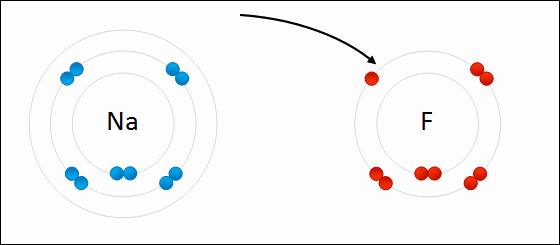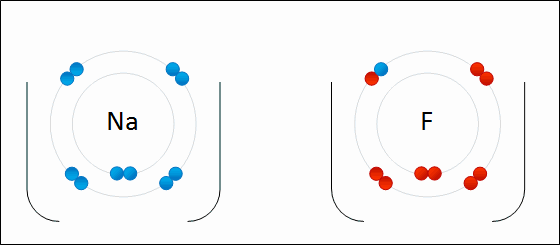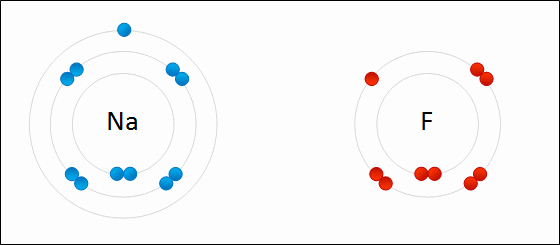
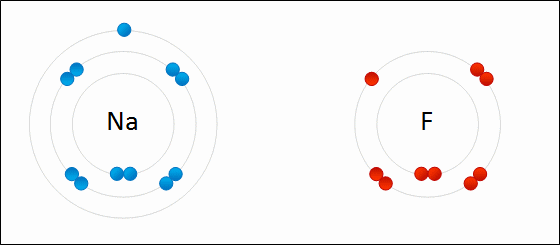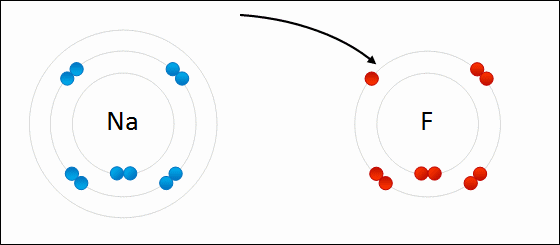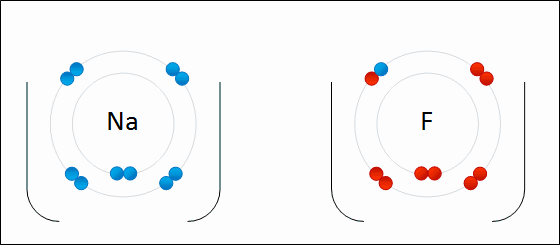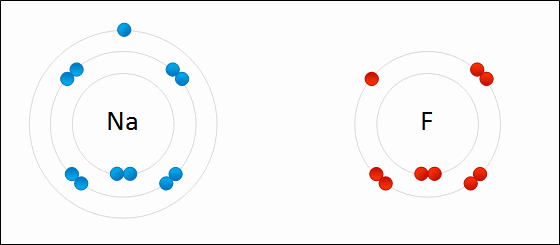
The bond formed when two or more atoms transfer electrons.
ionic bonding
Alkali metals will always ionize to form ions with a ____ charge.
1+
This type of covalent bond has an equal sharing of electrons.
Non-polar covalent bond.
Does Li₂O contain ionic or covalent bonds?
ionic
Ionic bonds form between what types of elements?
Metals and non-metals
If an atom loses an electron, then that atom will become ____ charged.
positively charged
Halogens always ionize to form ions with a ____ charge.
-1
Which periodic trend best explains why non-metals share electrons?
Electronegativity
Would Cl₂ have a nonpolar covalent bond or polar covalent bond?
nonpolar covalent bond
Does O₂ have a polar or non-polar bond?
Non-polar
If an atom gains an electron, then that atom will become ____ charged.
negatively charged

Alkaline earth metals always form ions that have a ____ charge.
+2
This type of covalent bond has an unequal sharing of electrons.
Polar covalent bonds
Would HBr have a polar covalent bond or nonpolar covalent bond?
polar covalent bond
What is the name of PCl₅?
Phosphorus Pentachloride
What specific type of electrons are responsible for the reactivities of the elements?
valence electrons
outermost electrons
electrons in outermost shell
These types of metals are located in the d-block.
Transition metals
Which bond is represented by 2 lines and shares a total of 4 electrons?
Double Bond
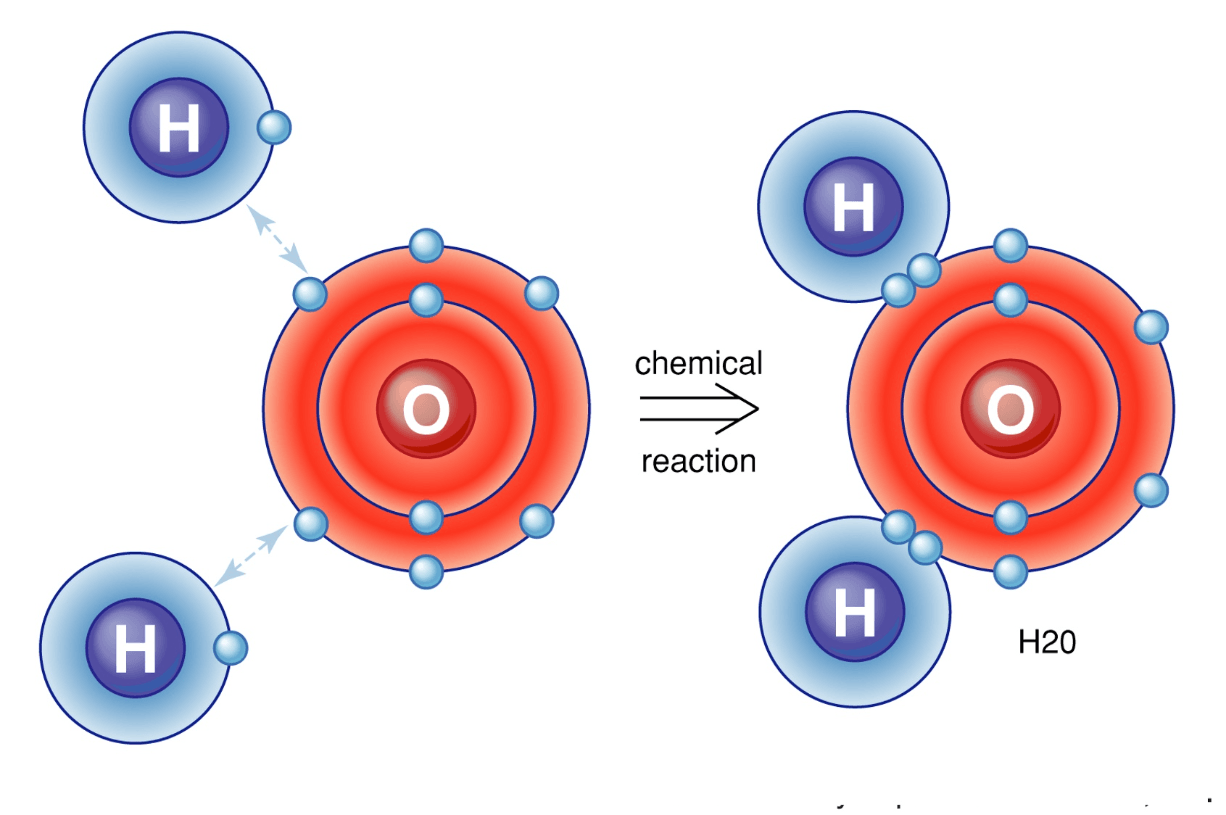
Is water (H₂O) polar or non-polar?
polar
What is the chemical formula for dichlorine heptasulfide?
Cl₂S₇