A reaction that involves breaking down a molecule into simpler substances
Decomposition reaction
Given the reactants B + AX, where AX is an ionic compound containing nonmetal X, what is are the products?
What is A + BX or X + AB ... which combination results depends upon whether B is a metal or a nonmetal
How do you determine the subscripts in molecules of products? (for example, if you know that the product contains Aluminum and oxygen, how do you know to write Al2O3?)
Use group # or transition metal name to find oxidation numbers for simple ions or a polyatomic ion chart for polyatomic ions; use crisscross rule.
Al 3+O2- Al2O3
Two elements reacting with each other OR two smaller chemicals reacting to form a larger, more complex chemical
What is synthesis/combination reaction
A + B --> AB
2FCl -> F2 + Cl2
Decomposition
remember that pure F and Cl both occur as diatomic molecules!
A reaction that gives off energy
Exothermic
A reaction that involves the combination of two or more substances to form a single compound
What is Synthesis or combination
Sc + O2 --> _________
scandium(III)
don't worry about balancing
What is Sc2O3... able to predict this due to format A + B-->AB showing that this is a synthesis (combination) reaction
What are the symbols of the 7 elements that are found in pure form as diatomic molecules?
H, N, O, F, Cl, Br, I
occur as H2, N2, O2, F2, Cl2, Br2, I2
Have No Fear Of Ice Cld Beer
Two ionic compounds react
Double replacement reaction
AB + CD where A and C are metals or positive polyatomic ions & B and D are nonmetals or negative polyatomic ions
Hydrogen plus Chlorine -> HCl.
classify the reaction AND write the balanced chemical equation
Synthesis/Combination reaction
H2 + Cl2 --> 2HCl
Predict products of this reaction and balance the equation
CaCl2 + K2CO3 -->
AB + CD --> AD + CB so double displacement or double replacement
CaCl2 + K2CO3 -->CaCO3 + 2KCl
A reaction that involves replacing one atom in a compound with another atom.
What is Single Replacement
C7H14O2 + O2 -->
don't worry about balancing
CO2 + H2O
What are the 2 products of a metal carbonate when it decomposes due to heating?
metal oxide solid and carbon dioxide gas
A single compound reacts
Decomposition reaction
(two or more products will be smaller or less complex)
AB--> A + B
CH4 + __O2 ->__ CO2 + __H2O + heat
classify the reaction and balance the equation
Combustion reaction
CH4 + 2O2 -> CO2 + 2H2O + heat
Heat on the reactant side
Endothermic
Reaction involves an exchange of metals between two different compounds (although H usually acts as a nonmetal, in these reactions, it acts as a metal)
What is Double Replacement
FeCl3 + BaS -->
don't worry about balancing
Fe2S3 + BaCl2
Why? AB +CD --> AD + CB double displacement or double displacement where the metals trade partners
The Law of Conservation of mass states:
The mass of the products must equal the mass of the reactants; the number of atoms of every element are equal on the reactants and products side of the equation.
A covalent molecule containing C and H (and perhaps other atoms, too) reacts with diatomic oxygen
Combustion reaction
__AgNO3(aq) +__Cu(s) ->__Ag(s) + __Cu(NO3)2(aq)
classify the reaction and balance it
Single Replacement
2AgNO3(aq) +Cu(s) ->2Ag(s) + Cu(NO3)2(aq)
Exo or endothermic?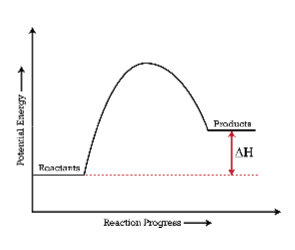
Endothermic (more energy in products that reactants, so energy had to be absorbed--added to--the reactant side of the equation)
A rapid reaction of a covalent molecule containing at least C and H with molecular oxygen to produce large amounts of heat, carbon dioxide gas, water vapor... usually produces a flame
What is Combustion
Given the products Carbon Dioxide + Water, give the general name of the reactants and what type of reaction this is.
What is Hydrocarbon, carbohydrate, or alcohol AND Oxygen;
Combustion
Classifyas endothermic (heat absorbing) or exothermic (heat releasing):
1) gets colder
2) gets hotter
3) A + B + energy --> AB
4) AB --> A + B + energy
1) gets colder endothermic
2) gets hotter exothermic
3) A + B + energy --> AB endothermic
4) AB --> A + B + energy exothermic
The law of conservation of energy states that the total energy possessed by reactants is ...?
equal to the total energy possessed by products
classify this reaction and balance the equation
__Fe2(CO3)3 --> Fe2O3(s) + ___CO2(g)
Decomposition of a metal carbonate (in the presence of heat)
Fe2(CO3)3 --> Fe2O3(s) + 3CO2(g)
Which letter represents the "Energy of Activation" (energy required to start any reaction, whether it is exothermic or endothermic)?
Is this an exothermic or endothermic reaction?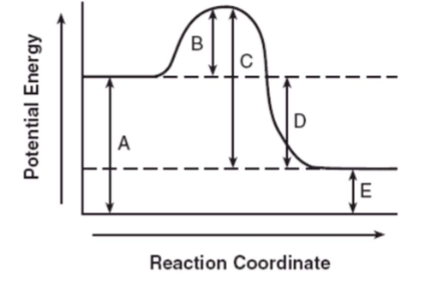
B
exothermic--since products have LESS energy than reactants, then the products had to release energy to the environment.