One of the following is NOT a physical change: splitting wood, melting ice, burning wood or dissolving salt in water.
Burning wood
Substance(s) that enters a chemical reaction is called this (on the left side of the reaction).
What is a reactant?
Balance the reaction: Na + H2O -> NaOH + H2
What is 2Na + 2H2O -> 2NaOH + H2
What is the precipitate in this reaction?
AgBr - silver bromide
The reaction 2HgO -> 2Hg + O2 is an example of this type of reaction.
What is decomposition?
A solid that forms from a solution during a chemical reaction.
Precipitate
What are the products in this chemical reaction?

NH3 - ammonia
Balance the reaction: HCl + Ca(OH)2 -> CaCl2 + H2O
What is 2HCl + Ca(OH)2 -> CaCl2 + 2H2O
Write the products of this single replacement reaction. Don't balance. Would the reaction occur?
Al + HCl -->
--> AlCl3 + H2
Yes, this reaction would occur

Single replacement
Give 3 signs that a chemical reaction has taken place.
Temp change, light produced, color change, the formation of gas, and the formation of a precipitate.
Write the 7 diatomic elements, including subscripts
H2 O2 F2 Br2 I2 N2 Cl2
In the formula 5CaSO4, this is the number of oxygen atoms.
What is 20?
Predict the products. Don't balance. Would this reaction occur?
Na2S(aq) + KOH(aq) -->
--> NaOH(aq) + K2S(aq)
No, both products are aqueous
What reaction type produces a precipitate?
Double replacement
What is the term used for "dissolved in water"
Aqueous
What symbol is placed over the arrow to indicate that heat is applied to a reaction?

Balance the reaction: C3H8 +O2 -> CO2 + H20
What is C3H8 + 5O2 -> 3CO2 + 4H2O
Aluminum is combined with oxygen gas. What is the product? Hint: Combine the two elements and balance the charges.
Al + O2 -- > ?
Al2O3
What type of reaction is this?
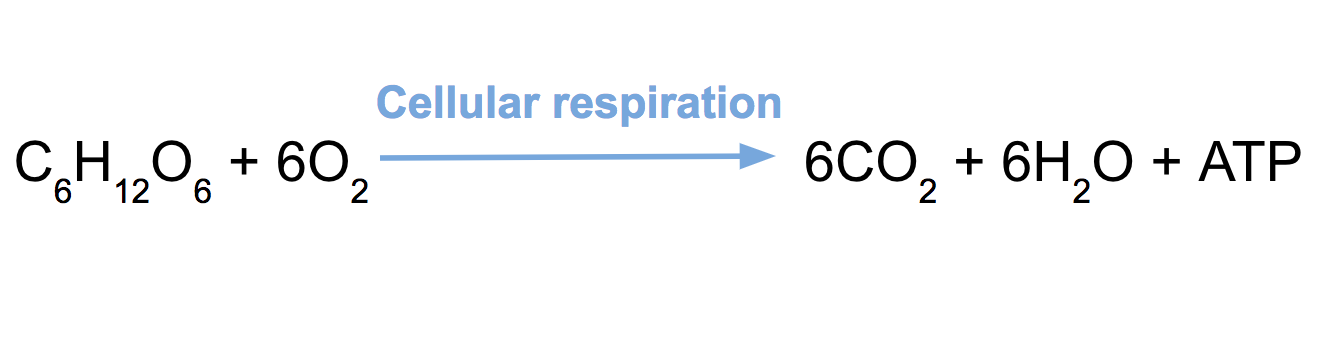
Combustion