Vince has a piece of metal and says it is made of only one element. Which of the following would best support his claim?
A) Every atom in the metal has 24 protons.
B) All of the atoms in the metal are vibrating at the same speed.
C) Some atoms in the metal have 24 protons, and others have 79 protons.
D) Some atoms in the metal are vibrating faster than others.
A) Every atom in the metal has 24 protons.
Which of the following is an example of a physical change?
(A) Iron rusting
(B) Paper burning
(C) Water freezing
(D) Bread baking
(C) Water freezing
When vinegar is mixed with baking soda, bubbles form. This shows that:
(A) A physical change is occurring
(B) A new substance is forming
(C) A solid is melting
(D) A gas is dissolving
B) A new substance is forming
In the reaction: Fe + O₂ → Fe₂O₃, what is needed to balance the equation?
a) Add coefficients to make atoms equal on both sides
b) Add more oxygen
c) Change the product
d) Remove iron atoms
a) Add coefficients to make atoms equal on both sides
Which of these is a physical change that takes place in a garden?
a. Insects eat the leaves of a plant for food.
b. Earthworms loosen the soil as they travel through it.
c. Leaves convert sunlight to sugar through photosynthesis.
b. Earthworms loosen the soil as they travel through it.
If an atom has 6 protons, how many electrons does it have in a neutral state?
6
A student mixes sand and salt and stirs the mixture. What type of change has occurred, and how can it be reversed?
(A) Chemical change; by heating
(B) Physical change; by using a magnet
(C) Physical change; by dissolving the salt in water and filtering the sand
(D) Chemical change; by adding vinegar
C. Physical change; by dissolving the salt in water and filtering the sand
What happens to the molecules during a chemical change?
(A) They change state but stay the same
(B) They rearrange to form a new substance
(C) They disappear
(D) They freeze
(B) They rearrange to form a new substance
What are the products of this reaction?
CaCO₃ → CaO + CO₂
a) Calcium carbonate
b) Calcium and carbon
c) Calcium oxide and carbon dioxide
d) Carbon dioxide and calcium hydroxide
c) Calcium oxide and carbon dioxide
Hydrogen gas reacts with oxygen gas to form water:
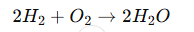
Question:
If you start with 10 g of hydrogen and an unknown mass of oxygen, and the reaction produces 90 g of water, what was the mass of oxygen used?
80 g of oxygen was used.
An unknown element has 11 protons, 12 neutrons, and 11 electrons.
What is its atomic mass and which element is it?
Atomic mass = 11 + 12 = 23
Element = Sodium (Na)
Which process involves both physical and chemical changes?
(A) Ice melting
(B) Cutting vegetables
(C) Frying an egg
(D) Water evaporating
(C) Frying an egg
A student performed a science experiment by mixing 60 grams of Liquid X with 150 grams of Liquid Y in a beaker. A chemical reaction occurred, creating gas bubbles and forming a new liquid. Both liquids were completely used up in the reaction. What should be the total mass of the gas and the new liquid after the reaction?
A) 10 grams
B) 60 grams
C) 90 grams
D) 210 grams
D) 210 grams
A student pours two different liquids into a 55-gram beaker. Each liquid has a mass of 45 grams. When mixed, a chemical reaction occurs. After the reaction is finished, the beaker is placed on a scale, and the total mass reads 80 grams. Which explanation best describes why the mass is less than expected?
A) Some of the mass was converted into heat during the reaction.
B) Some of the mass was converted into energy during the reaction.
C) Some of the mass remained trapped inside the substances in the beaker.
D) Some of the mass escaped as a gas during the reaction.
D) Some of the mass escaped as a gas during the reaction.
A student conducts a reaction in an open beaker and notices that the mass before the reaction was 50 g, but after the reaction, it was only 45 g.
Question:
Did the student violate the Law of Conservation of Mass? Why or why not?
No, the Law of Conservation of Mass was not violated—it just wasn’t a closed system.
Why are the elements in Group 18 (Noble Gases) considered unreactive?
Because their outer electron shells are full
If a physical change occurs, which of the following must always be true?
(A) The substance will emit light
(B) A new substance is formed
(C) The change is reversible
(D) The chemical composition of the substance stays the same
(D) The chemical composition of the substance stays the same
A student lights a candle, and it burns for several minutes. The student makes these two observations:
Observation 1: The candle wick, which is white, turns black as it burns.
Observation 2: The solid wax near the flame melts into a liquid.
Which option best explains the type of change happening in each observation?
A) Observation 1 is a physical change, and Observation 2 is a chemical change.
B) Observation 1 is a chemical change, and Observation 2 is a physical change.
C) Both observations are physical changes.
D) Both observations are chemical changes.
B) Observation 1 is a chemical change, and Observation 2 is a physical change.
In a chemical reaction, the mass of the products is always...
a) Less than the mass of the reactants
b) More than the mass of the reactants
c) Equal to the mass of the reactants
d) Unpredictable
c) Equal to the mass of the reactants
During a lab, magnesium is burned in air to form magnesium oxide. The mass of magnesium used was 5 g, and the final mass of magnesium oxide was 8.3 g.
Question:
Where did the extra mass come from, and how does this support the Law of Conservation of Mass?
The extra 3.3 g came from oxygen in the air.
Explanation:
Magnesium reacts with oxygen in the air. Though oxygen wasn't weighed before, it was part of the reaction. So:
5 g (Mg) + 3.3 g (O₂) = 8.3 g (MgO)
This supports the Law of Conservation of Mass: nothing is lost; it just changes form.
Given a group in the periodic table, for example, alkali metals, what happen to the reactivity of elements as you move down the group?
Increases as you move down the group
Dry ice (solid CO₂) changes directly into gas at room temperature. Which best describes this process?
(A) It's a chemical reaction
(B) It's a physical change called sublimation
(C) It's a physical change called condensation
(D) It's an irreversible chemical change
(B) It's a physical change called sublimation
In the reaction shown:
SiO2 + 2C --- Si + 2CO
solid silicon (Si) forms and collects at the bottom of the furnace, while carbon monoxide (CO) is released as a gas and escapes into the air. Based on these observations, which statement is best supported?
A) The reaction took place in a closed system.
B) The reaction took place in an open system.
C) The total mass of the products is less than the total mass of the reactants.
D) The total mass of the products is greater than the total mass of the reactants.
B) The reaction took place in an open system.
Does the chemical equation:
SiO2 + 2C ----- Si + 2CO
support the law of conservation of mass?
A) No, because it shows more silicon (Si), oxygen (O), and carbon (C) atoms in the reactants than in the products.
B) No, because it shows more molecules in the products than in the reactants.
C) Yes, because it shows an equal number of Si, O, and C atoms in both the reactants and the products.
D) Yes, because it shows an equal number of molecules in the reactants and products.
D) Yes, because it shows an equal number of molecules in the reactants and products.
A student mixes 12 g of baking soda with vinegar in a sealed container. A chemical reaction occurs, producing carbon dioxide gas. After the reaction, the total mass of the sealed container and its contents is still 112 g.
Question:
If the mass of the container and vinegar before the reaction was 100 g, what was the mass of carbon dioxide produced? Explain using the Law of Conservation of Mass.
0 g of carbon dioxide was lost; the mass of CO₂ is already included in the 112 g.
Baking soda: 12 g
Container + vinegar before reaction: 100 g
Total mass after reaction (sealed container): 112g