This is the "before substance" in a chemical reaction.
Reactant
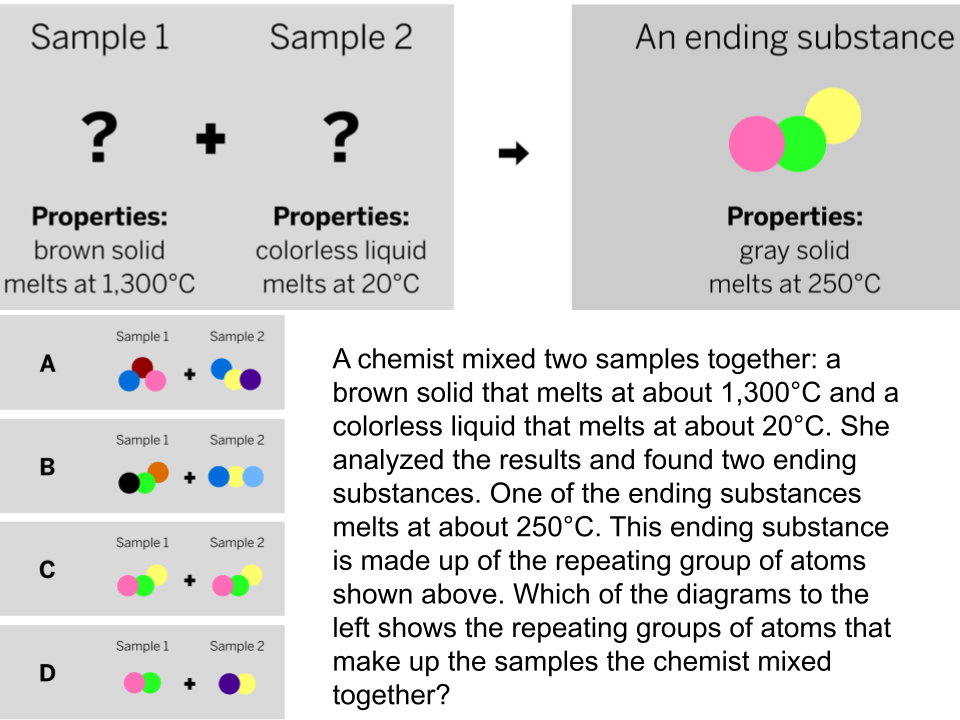
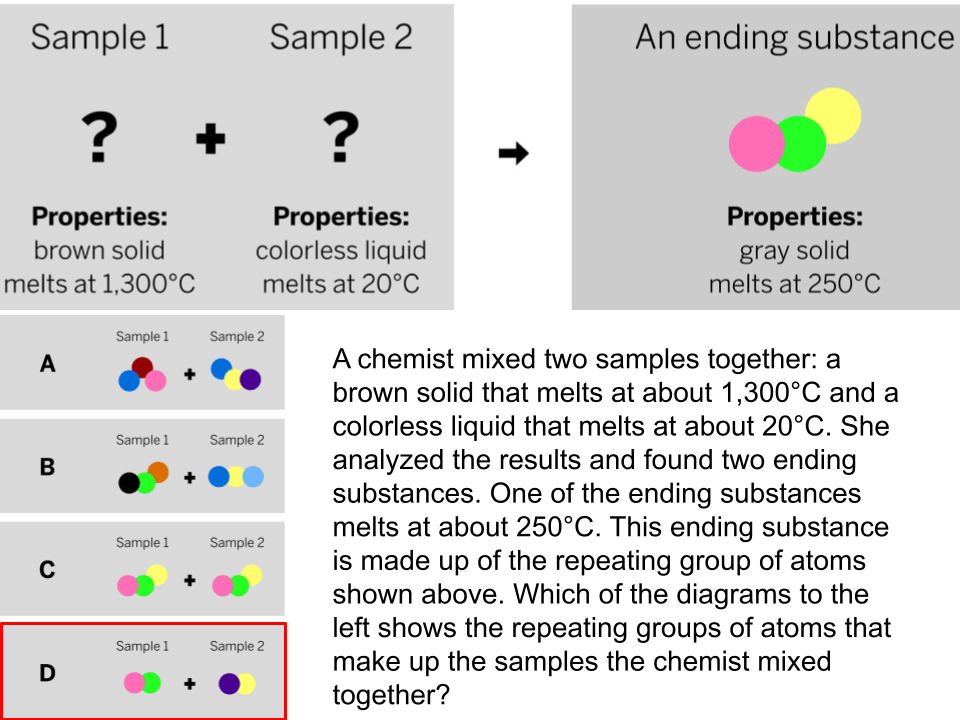
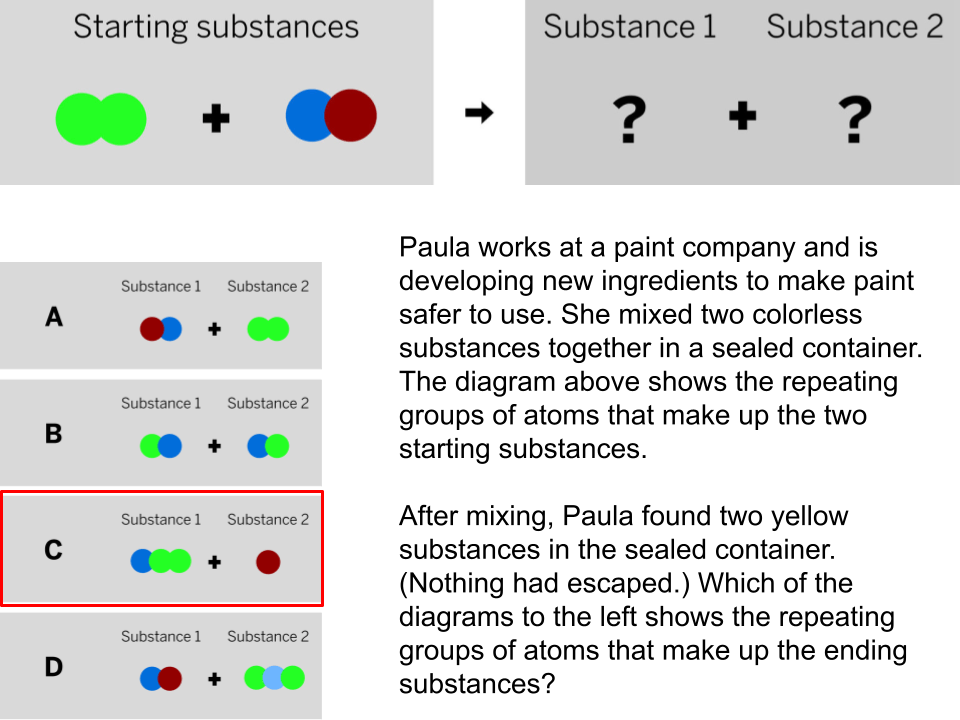
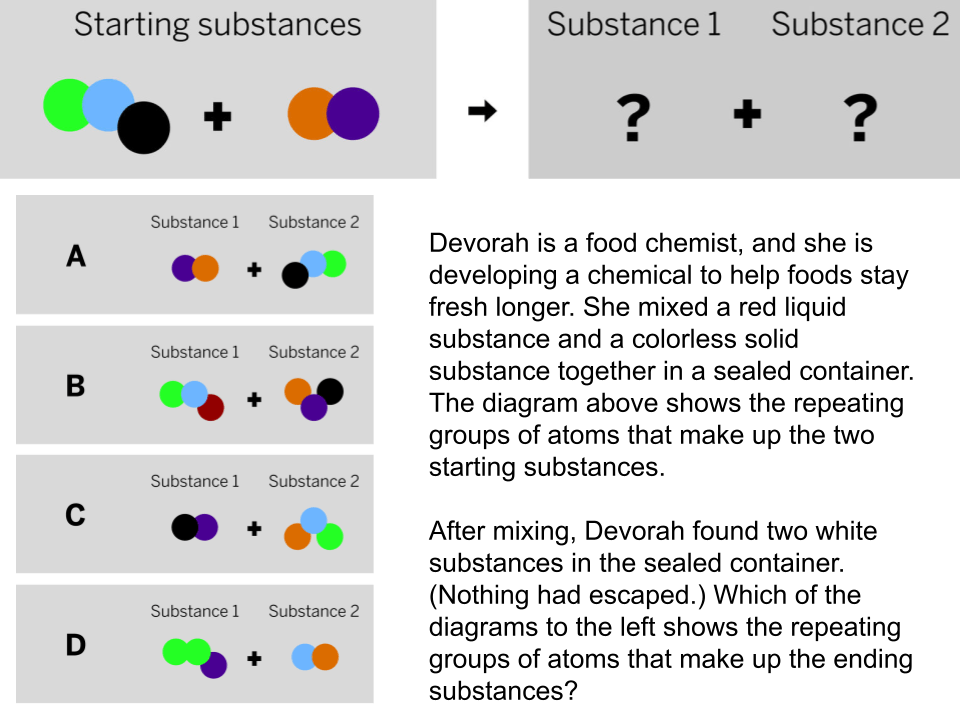
The diagram shows the repeating groups of atoms that make up two samples. Will the properties of the two samples likely be the same or different? (Explain)

The properties will likely be different because the repeating groups of atoms that make up each sample are different.
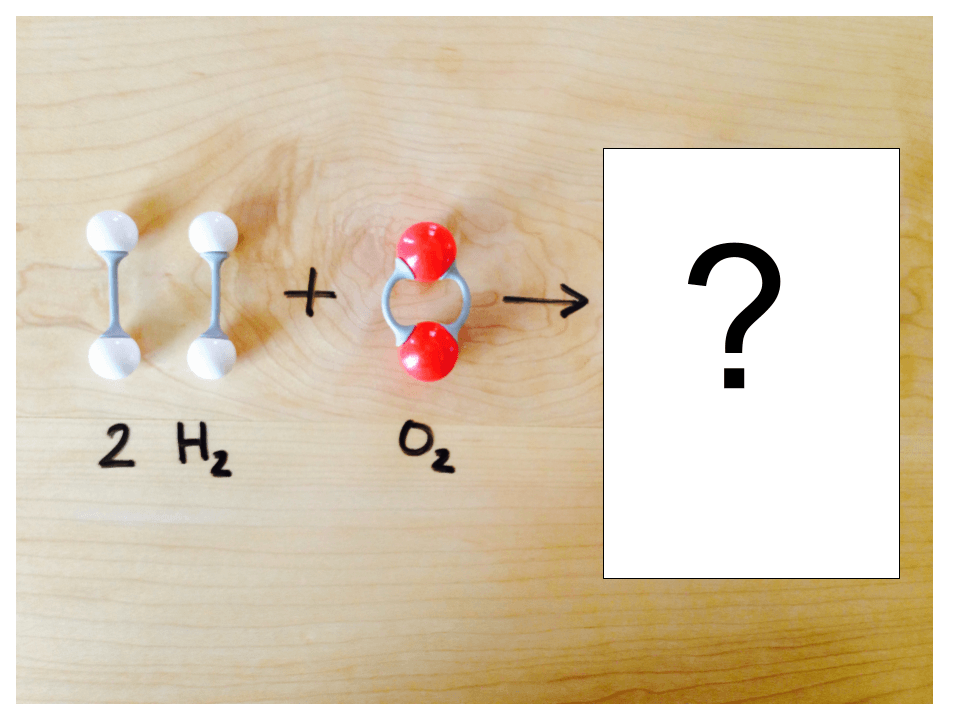
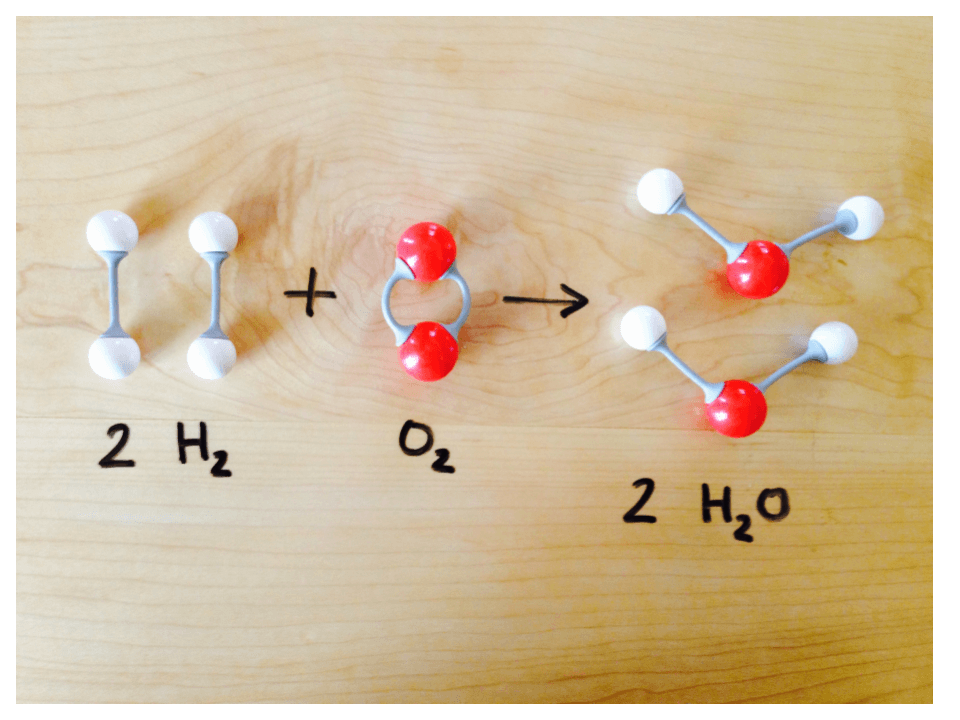
List three examples of properties a substance could have.
Color
Texture
Smell
Magnetism
Grain size
Phase at room temperature
ETC.
This is the "after substance" in a chemical reaction.
Product
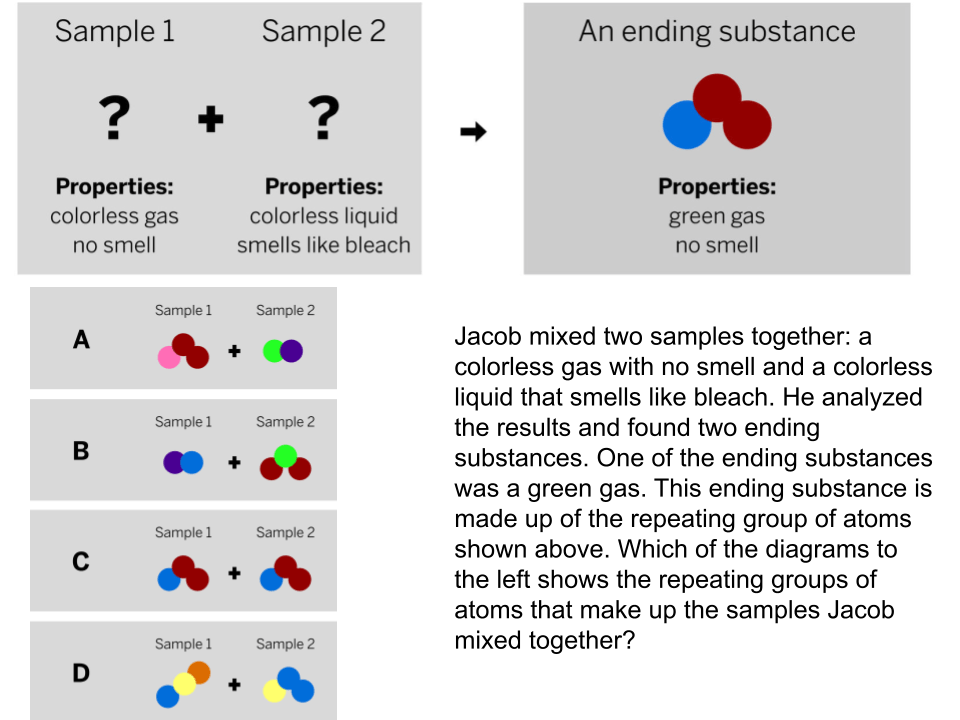
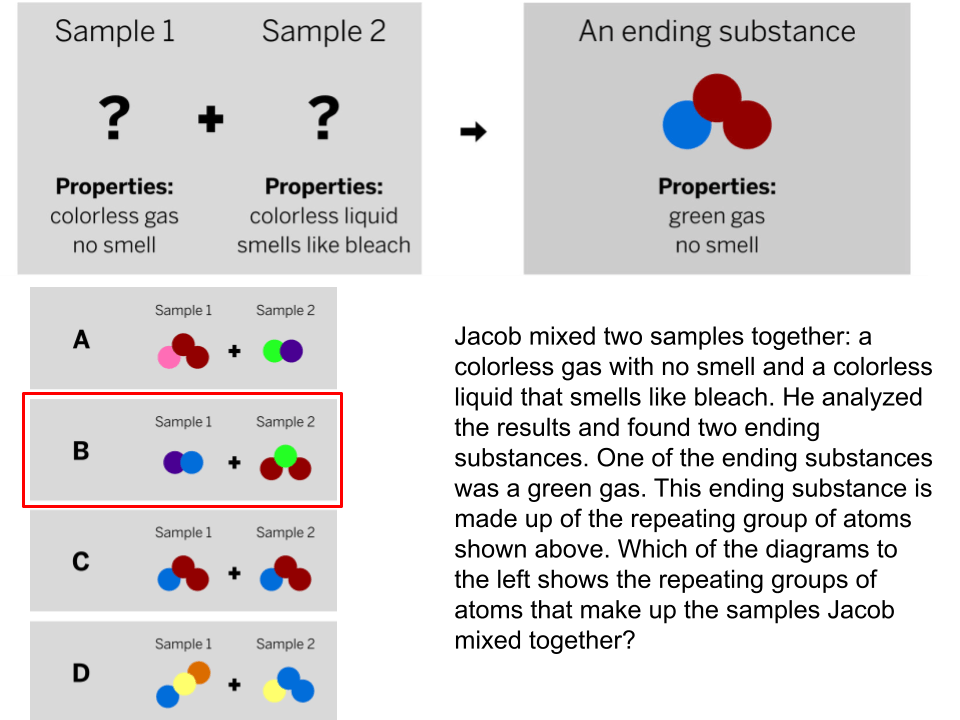
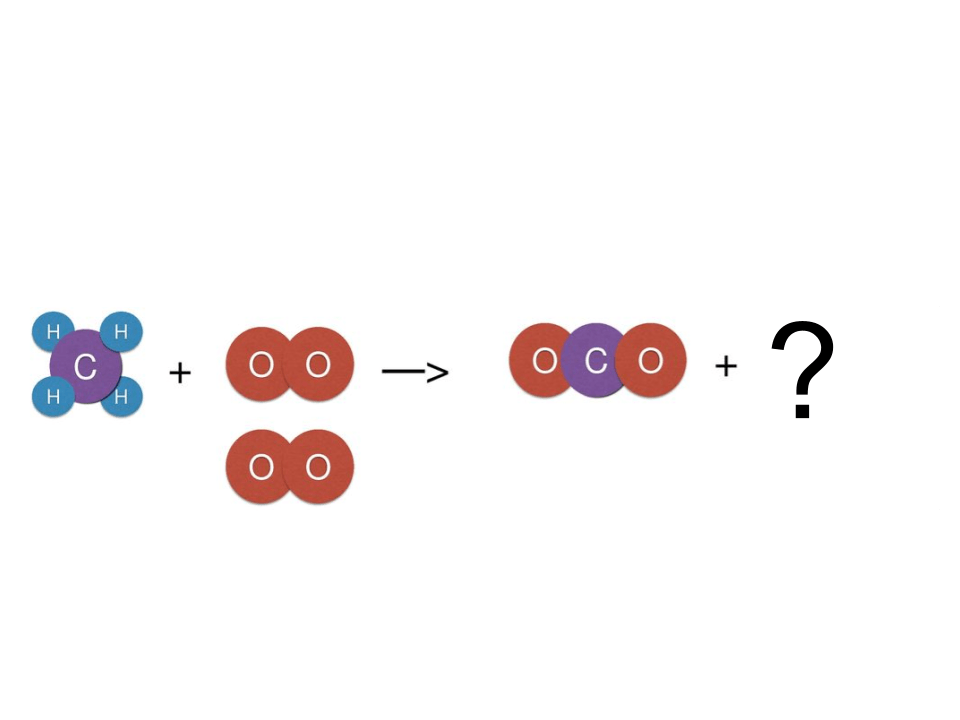

You mix a sour smelling clear liquid and an odorless white powder. A chemical reaction occurs. What will the properties of the product be?
Not enough information to determine.
(PROPERTIES DO NOT NECESSARILY TRANSFER - NEW SUBSTANCE = NEW PREOPERTIES!)
During a chemical reaction atoms ________ to form new molecules and repeating groups of atoms.
During a chemical reaction atoms REARANGE to form new molecules and repeating groups of atoms.
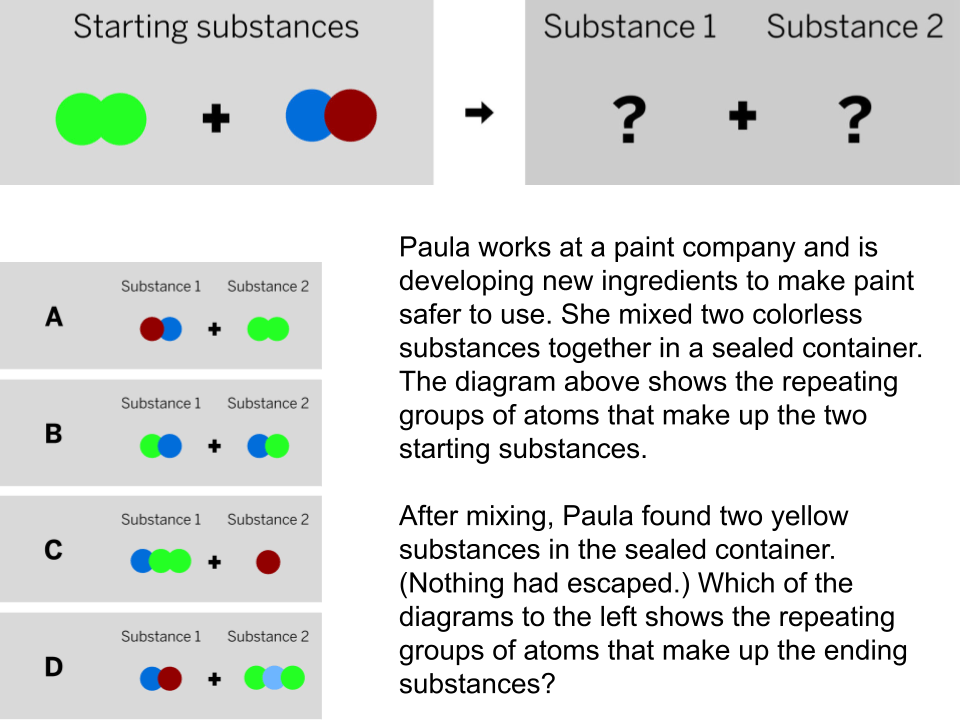
Free Points
True or false.
Properties are due to the arrangement, type or number of atoms within a substances repeating groups of atoms.
TRUE
This example of a property is the temperature at which a substance changes from the solid phase to the liquid phase.
melting point
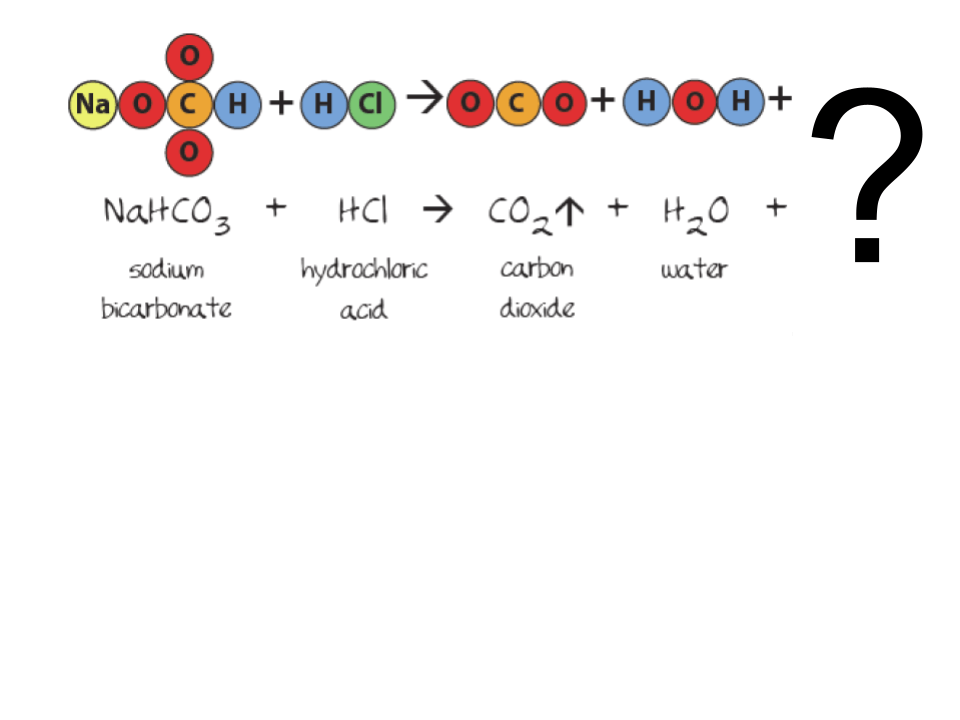
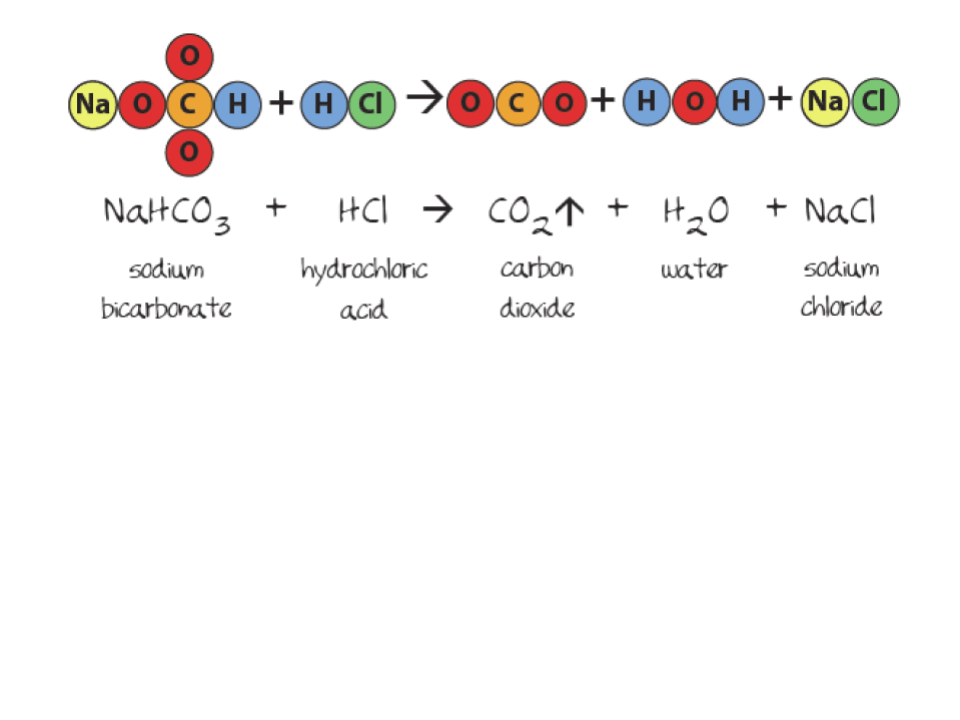
This example of a property is the temperature at which a substance changes from the liquid phase to the gas phase.
boiling point
Hydrofluoric acid was able to eat through the glass display case in the museum because it has this property.
corrosive
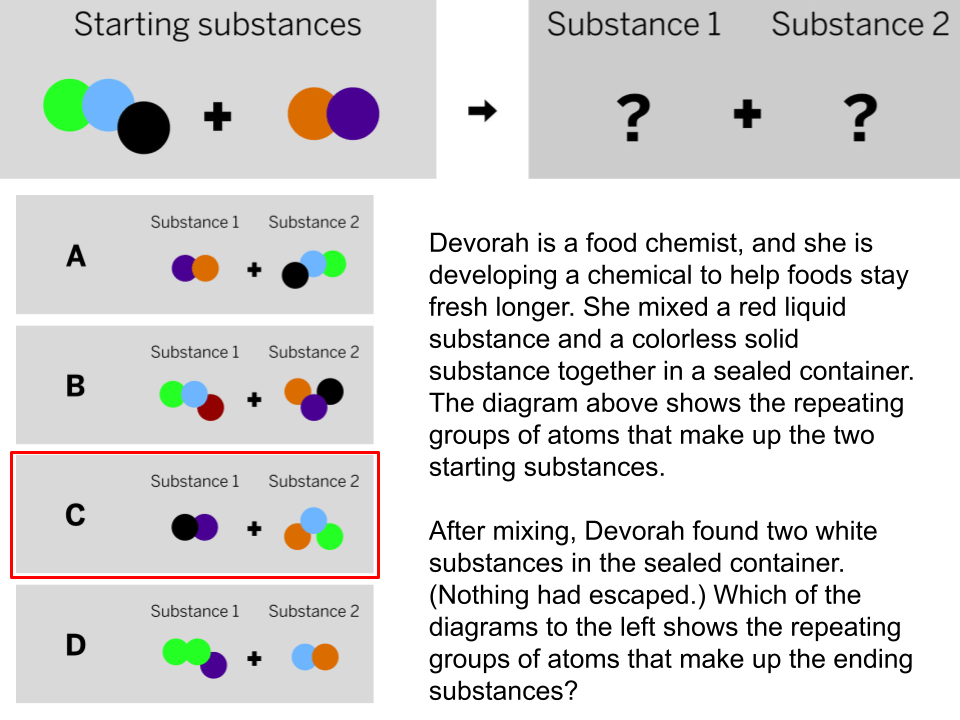
Free Points
Which of these is not a property:
Liquid at room temperature
Contains 2 hydrogen and 1 oxygen
Freezes at 32 degrees Fahrenheit
Clear
Contains 2 hydrogen and 1 oxygen
This is the atomic make up which determines the properties!