The smallest unit that all matter is made of
atom
List 3 signs of a chemical change.
new odor
gas is produced
precipitate (solid) forms
temperature changes
color change
Mass cannot be _________ or __________ but it can be rearranged.
created, destroyed
plants
soil
oil
water
etc.
List 3 possible physical properties.
smell, weight, boiling point, melting point, color
a group of atoms joined together in a particular way
molecule
What is one example of a chemical change that we observed in class together?
photosynthesis, foam gnome (poly A & B mixed together), baking soda & vinegar, cleaning a penny with salt/vinegar
How many carbon atoms are on the reactant side of this equation? Product side?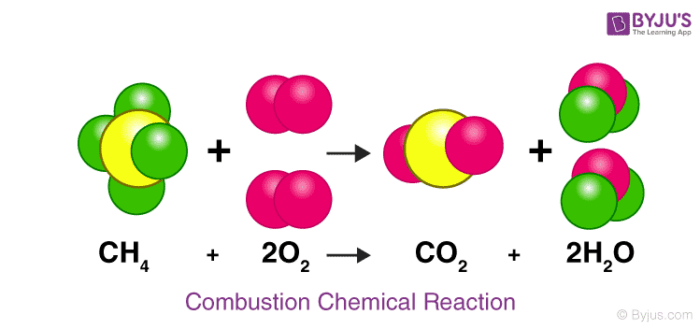
1, 1 (yellow)
What are 2 examples of a synthetic material?
nylon
plastic
biodiesel
etc.
flammable, dissolves in water, reacts with oxygen
the substance(s) that go INTO a chemical reaction; left side of the arrow
Reactant
What are the reactants in this chemical reaction?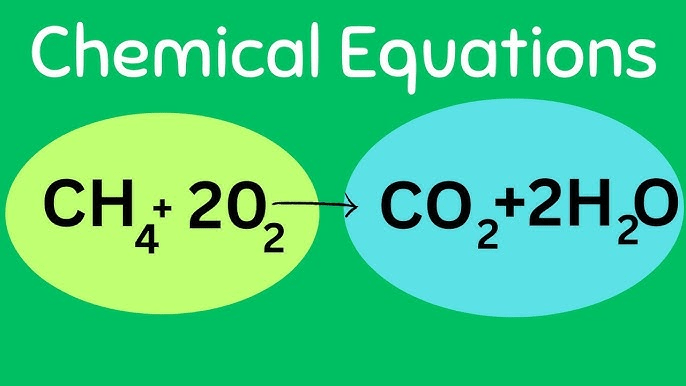
CH4 (methane) and O2 (oxygen)
How many oxygen atoms are the reactant side of this equation? Product side?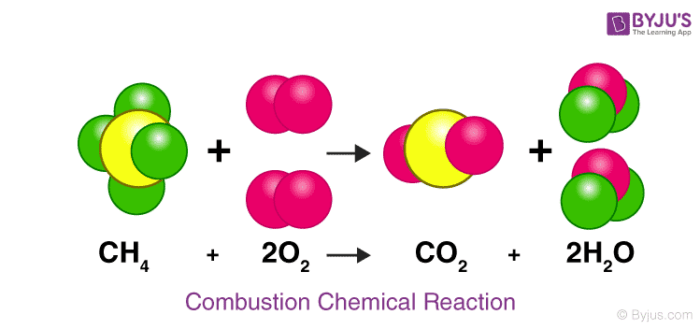
4, 4 (pink)
Wool is taken directly from sheep (nature) while nylon is a synthetic material derived from coal and petroleum getting chemically mixed together. Both of these products are made of what? (hint: vocab word)
Natural Resources
List 3 properties (chemical and/or physical) of water.
Boiling Point: 100 Celsius, 212 Fahrenheit
Melting Point: 0 Celsius, 32 Fahrenheit
Odorless
Colorless
Liquid at room temperature
Not flammable
define the law of conservation of mass
Mass cannot be created or destroyed but it can be rearranged.
What are the products in this chemical reaction?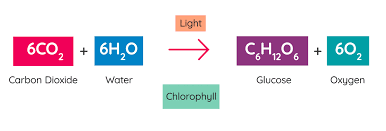
glucose and oxygen
How does this chemical reaction follow the law of conservation of mass? Explain your answer using the number of each type of atom on both sides.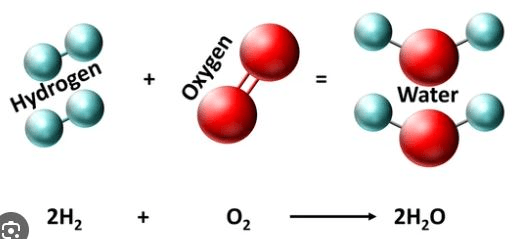
This follows the law because there are 4 hydrogen atoms on both sides and 2 oxygen atoms on both sides. This shows that mass was not created or destroyed, it was just rearranged.
Which of these is NOT a synthetic material? Explain.
Plastic, Oil, Nylon
Oil is not a synthetic material because it can be found in nature (it is a natural resource).
Think about the foam gnome experiment. How did the properties of reactants compare to the properties of the products? Be specific.

The reactants were both liquids with a syrup-y consistency. They were both a yellow or brown color and one had a mild odor. The product was a solid that had a much larger volume. It had no smell and was warm.
A chemical change creates a new substance (atoms are rearranged) while a physical change is only a change in the appearance of a substance; no atoms are rearranged.
You decide to roast a marshmallow but it catches on fire by accident. Is this a chemical or physical change? Explain in one sentence using at least 2 vocab words.
This is a chemical change because a new substance is made. The ATOMS of the marshmallow have been rearranged to form new MOLECULES which have different PROPERTIES than the original marshmallow.
How does this chemical reaction follow the law of conservation of mass? Explain your answer using the number of each type of atom on both sides.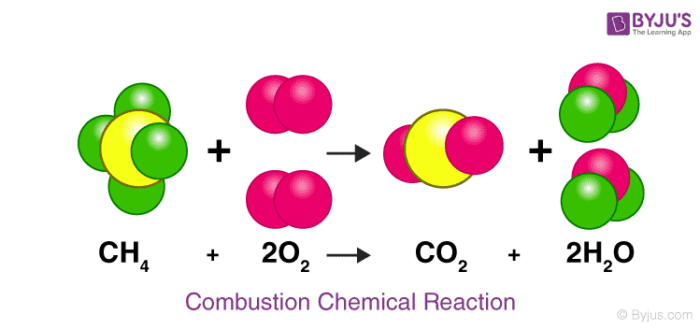
This follows the law because there are 4 hydrogen atoms on both sides, 4 oxygen atoms on both sides, and 1 carbon atom on both sides. This shows that mass was not created or destroyed, it was just rearranged.
Are synthetic materials good or bad for the environment? Defend your answer.
Synthetic materials often are more durable, which means they take a LONG time to break down. This can ultimately cause them to be harmful to the environment.
Describe the properties of the reactants of the baking soda/vinegar chemical reaction and compare them to the product.
The vinegar was a liquid at room temperature with a strong odor. The baking soda was a white powder at room temperature. The product was a gas (bubbles) and leftover vinegar and baking soda.