What is a reactant?
A chemical that is at the beginning of the reaction.
How many electrons does it take to fill up the first electron shell?
2
T/F - Catalysts are used up during a chemical reaction.
False
How many TOTAL atoms are in the following molecule:
C2H6
8
What is a valence electron?
Electrons in the outer shell (highest energy level) of an atom.
What is a catalyst?
Oxygen molecule.
Which side of this equation are the reactants?
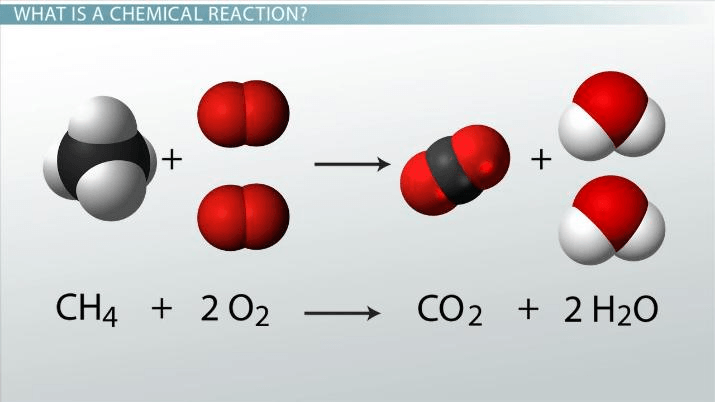
The Left
How many Nitrogens are in the following molecule?
2NH3
What does an atomic number tell you?
The number of protons and electrons in an atom.
What is a precipitate?
Electrons bond in order to fill up their:
Outer Shell (or energy level)
List 2 signals that a chemical reaction has taken place.
Bubbling
Color Change
Temperature Change
Forming of a Precipitate
etc.
How many total MOLECULES are on the reactant side of the following chemical equation?
CH4 + 2O2 = CO2 + 2H2O
3
T/F - Group 18, the noble gases, all have full valence shells. This means they do not readily bond with other atoms.
True.
What is a cation?
A positively charged ion
Why is this the correct Lewis Dot Structure of Carbon, even though it has 6 electrons?
We only draw the valence electrons.
T/F - the only conditions that every chemical reaction needs are the reactants getting near each other.
False - temperature (or other energy like light) must be high enough.
T/F - the number of atoms and molecules on both sides of a chemical equation are equal.
False
What is a polar molecule? List an example of one.
A molecule that has a slightly positive end and a slightly negative end.
Ex:H2O
What is the conservation of mass?
The conservation of mass states that the amount of mass (number of protons, neutrons, and electrons) will always be equal on both sides of a chemical equation.
What is the difference between ionic and covalent bonding?
Ionic bonds occur between a metal and a non metal. They give and take electrons, forming ions.
Covalent bonds occur between two non metals. They share electrons and do not form ions.
T/F - testing with different chemicals (and performing different chemical reactions) can help you identify an unknown substance.
True
How many total atoms are there in the following molecule?
H2CrO4
7
T/F Ice melting is a chemical reaction.
False