What is matter?
It is things that has mass and volume
Name the three subatomic particles within an atom
Proton Neutron Electron
In the periodic table, elements in the vertical columns form this
Families or Groups
Isotopes have the same ____ but different ___.
atomic number (number of protons), mass number (number of protons + neutrons)
The letter H in the Periodic Table stands for this element.
Hydrogen
What is mass?
It is the amount of "stuff" in an object
Where are protons located?
The nucleus
The horizontal rows on the periodic table are called
Periods
True or False... Isotopes of an element have different number of neutrons.
TRUE
The letter B in the Periodic Table stands for this element.
Boron
The smallest unit of an element is a what?
An atom
What does the atomic number tell us?
The number of protons in the nucleus.
The elements in group 18 are known as the--
Noble Gas
Why are Carbon-12 and Carbon-14 isotopes?
They have the same atomic number (number of protons).
The sum of the number of Protons plus Neutrons in the Nucleus.
The Atomic Mass
What is an element?
A pure substance that cannot be broken down.
Where are neutrons located?
In the nucleus
Elements that are good conductors of electricity are called
metals
Why are these two atoms not isotopes
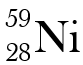
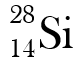
They have different atomic numbers.
This is a chart which organizes the chemical elements.
The Periodic Table
Draw the different formations of atoms in solids, liquids and gases
Solids- tight together
Liquid- more spread apart
gas- far apart.
Protons + Neutrons = ____________
Protons + Neutrons = the mass number of an atom
What number is the element Hydrogen?
1
True or False... Atom A has 13 protons and 14 neutrons. Atom B has 14 protons and 13 neutrons.
Atom A and Atom B are isotopes.
False. Isotopes have the same number of protons.
Give the group number and period of Gold
11, 6