The charge of a neutron
What is neutral?
He discovered the nucleus of an atom
Who is Ernest Rutherford?
Anything that has mass and takes up space
What is matter?
Balance this equation:
H2 + O2 -----> H20
What is 2,1,2
One or more elements that are chemically bonded together
What is a compound?
The location of electrons
What is the atomic shells/levels/ orbiting around the nucleus?
All atoms of zinc have this number of protons
What is 30 protons?
Matter can neither be created nor destroyed
What is the Law of Conservation of Mass?
Balance this equation
Ag2O --> Ag + O2
What is
2,4,1
Name this Compound
CO2
What is Carbon Dioxide?
The subatomic particle with the smallest mass
What is electrons?
The atomic number refers the the number of this subatomic particle
What is number of protons in the nucleus?
A simplified model of the atom
What is the Bohr Model?
balance the equation
P+ Cl2 → PCl3
When atoms share electrons
What is covalent bond?
The period and group number of Carbon
One of two or more atoms that have the same atomic number but a different number of neutrons.
What is isotopes?
A Chemist's most important tool in learning the behavior and properties of elements
What is the Periodic Table?
Balance the equation
FeCl3 + NaOH --> Fe(OH)3 + NaCl
What is 1,3,1,3?
When one or more electrons from one atom are removed and attached to another atom.
What is ionic bond?
The reason Helium only has 2 valence electrons but is located in Group 8
What is Helium only has one shell so the max valence electrons it can have is 2. It has a full outer shell and meets the requirement of being non-reactive like the other Noble Gases?
This is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving the atom or molecule a net positive or negative electrical charge.
What is ion?
Most matter on Earth has an overall charge of
What is neutral?
Name this element
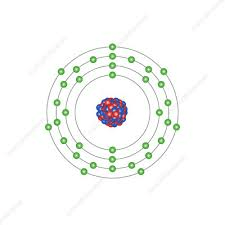
What is Bromine?
The Atomic Number of Oxygen is 8, what would happen if I added one more proton to it's nucleus?
What is you would no longer have Oxygen? You'd have Fluorine.