How many chemical bond types are there?What are they?
3
Ionic, Covalent, Metallic
In binary compounds, the metal always comes first True/False
True
Which bond is the strongest? single, double or triple
3
What type of bond will form between (Fe) and (O)
Ionic
What is the chemical formula for ammonia?
NH₃
What are alloys, and are they stronger or weaker than the elements used to make them?
They're two metals combined by being melted together. They're stronger.
How would you write (boron trifluoride) in a formula
BF₃
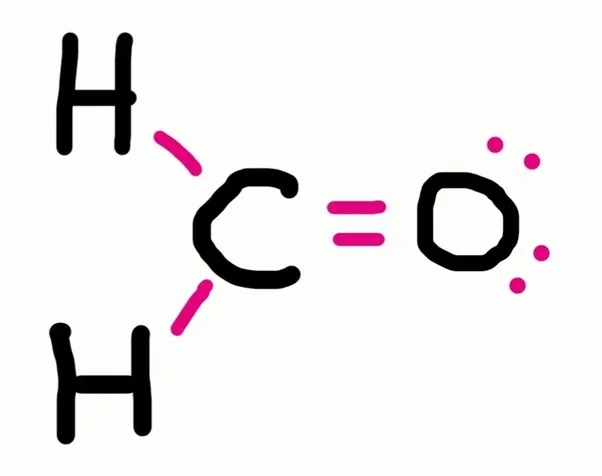 Write this as a formula?
Write this as a formula?
CH₂O
Since carbon has 4 valence electrons it will always be the central atom in a molecule. True/False
False
Chemical name for K₂S
Potassium Sulfide
Electrons and the nucleus attract each other because of __________ attraction?
Coulombic
When a binary compound has one transition metal do roman numerals or prefixes come in to play?
Roman numerals
Other than "Lewis structures", Lewis Dot diagrams are also known as ________.
Electron Dot Diagrams or Electron Dot Structures
Which has a greater electronegativity (Cl⁻), (Cl), or (Cl⁺) and why?
(Cl⁺). Because it lost an electron electrons are more drawn to the nucleus thus increasing its electronegativity.
What is the name of this formula CaCO₃
Calcium Carbonate
What is the strongest bond out of covalent, ionic, and metallic.
Covalent
When writing out a formulas name prefixes 1-10 can be used even on the 1st element?
true/false
False, you can not use mono on the first element
Lewis Structure is only used for what type of bond?
Covalent
Ionization energy decreases across a period and increases down a group?
True/False
False since its the exact opposite Explanation:Ionization energy increases across a period and decreases down a group?
Formula for Dihydrogen oxide
H₂O
List 4 out of 7 of the Super Seven diatomic elements.
any 4 of these would have worked (N, O, F, Cl, Br, I, At)
There's 6 nitrogen atoms in Al(NO₂)₃ True/False
False Explanations: There's 2
What group of elements could you use to replace Carbon in this CH₂O
Group 14
What is the ratio of the formula (ClO), and the chemical name?
1:1, and Ch
If we combined aluminum with chlorine what will be the ratio of Al to Cl. Hint: Al has a positive charge of 3 while Cl has a negative charge of 1
1:3