&
Compounds
The smallest unit of matter which has properties of a chemical element.
What is an atom?
These are the ingredients of a reaction; they are on the left side of the arrow.
What are reactants?
Which represents a compound?
What is B?
Is this chemical equation balanced?
NaCHO3 + 3HC2H3O2 ---> NaC2H3O2 + 3H2O + CO2
What is not balanced?
Anything that has mass and takes up space?
What is matter?
Carbon & oxygen combine chemically to form carbon dioxide. What is carbon dioxide?
What is a compound?
These are the end results of a reaction; they are on the right side of the arrow.
What are products?

Which number represents as element?
What is number 2?
In a ____________ system, all the matter stays inside.
What is closed?
Matter can not be created nor destroyed: it can only be?
What is transformed or changed?
Oxygen is what kind of matter?
What is an element
The symbolic representation of a chemical reaction using symbols and chemical formulas.
What is a chemical equation?
What is this particle diagram showing?
What are elements?
If reaction starts with 20g of reactants it should produce?
What is 20g of products?
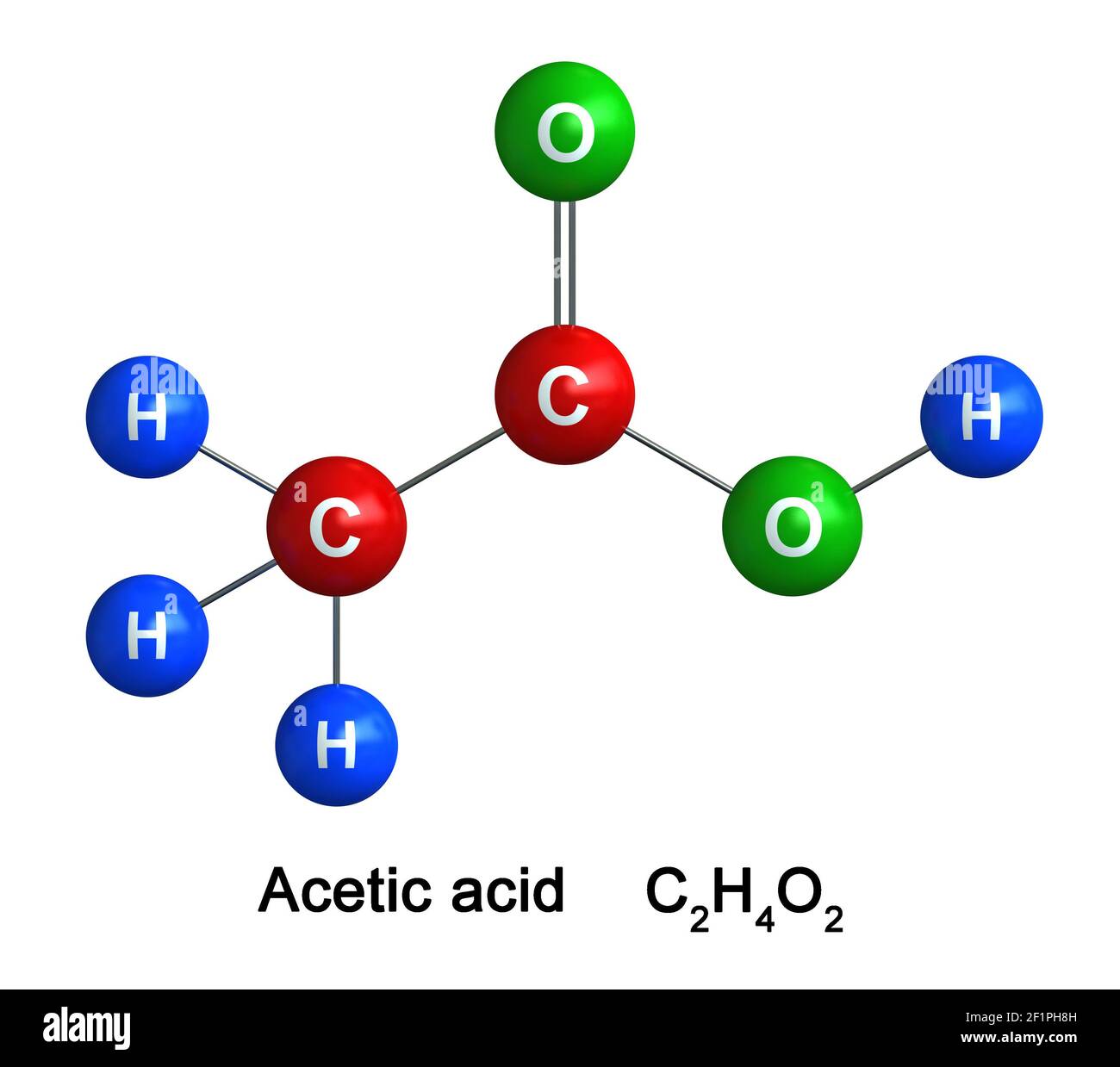
Draw the molecule below:

What is....?
Soil and air are examples of what type of mixtures? (answer in the correct order)
What are heterogeneous and homogeneous mixtures.
The large number in front of a molecule is called the ________. It tells us the number of molecules in an equation.
What is coefficient?
What is this particle diagram showing?
What is a mixture of compounds?
Is this equation balanced or unbalanced?

What is balanced?
If a container does NOT have a lid, it is called a what system?
What is open?
What's the difference between an element and a compound?
What is elements cannot be broken down into simpler substances, while compounds can.
The number placed after a symbol in a chemical reaction is
What is a subscript?
What is this particle diagram showing?
What is a mixture of elements and compounds?
What happens to the total mass of the substances after a chemical reaction?
What is stays the same?
To follow LCOM, there should be the same number of ____ for each element on both sides of the equation.
What is atoms?