The atomic mass and the atomic number.
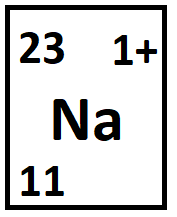
What are 23 and 11?
A = 23
Z = 11
The Periodic Table is organized by this.
What is increasing atomic number?
This is always written first in the formula of an ionic compound.
What is the metal?
NaCl MgCl2 AlCl3
The type of reaction
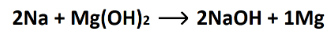
What is a single replacement reaction?
The relative mass and charge of an electron.
What is 0 and -1?

The number of protons, neutrons, and electrons in the following ion:
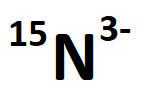
What are 7 protons, 8 neutrons, and 10 electrons?
The number of neutrons in the following ion:
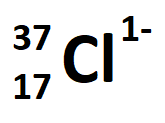
What is 20?
The name given to Group 2 elements.
What are the Alkaline Earth Metals?
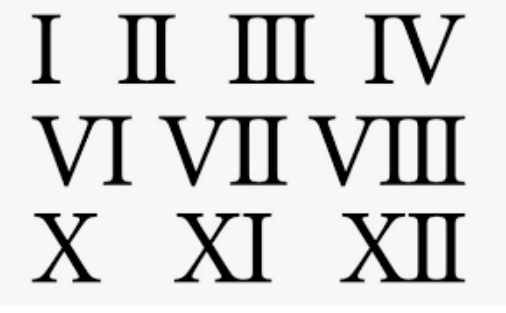
These must be used when naming compounds that include transition metals.
What are Roman Numerals?
The products of this reaction:
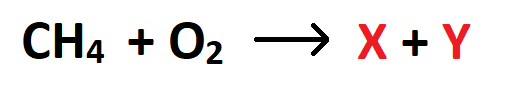
What are carbon dioxide and water?
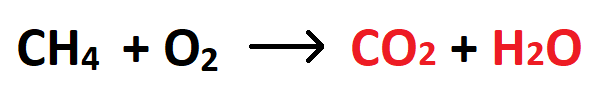
The density of water, including units.
What is 1 g/ml?
The name of Fe3(PO4)2
What is iron (II) phosphate?
The number of protons and the number of electrons.
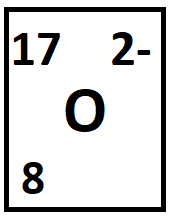
What are 8 and 10?
p = 8
e- = 8 + two extra electrons = 10
These elements in the Periodic Table:
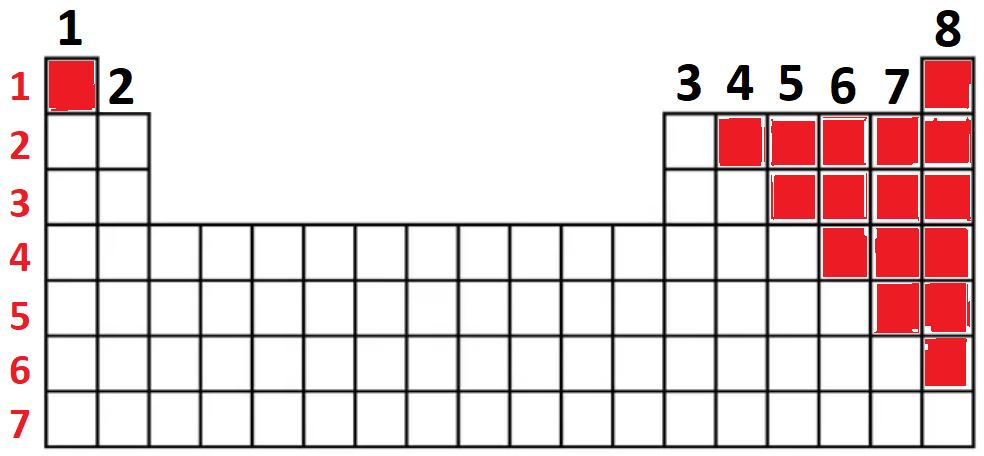
What are the non-metals?
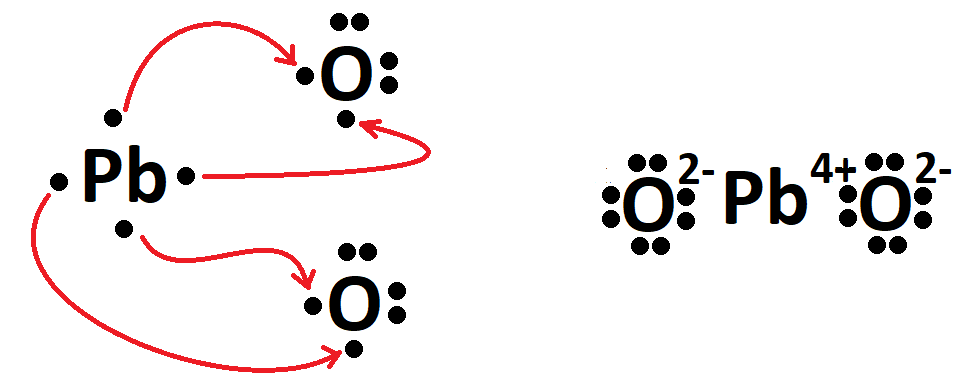
The formula of lead (IV) oxide.
What is PbO2?
The name given to the numbers used to balance equations:
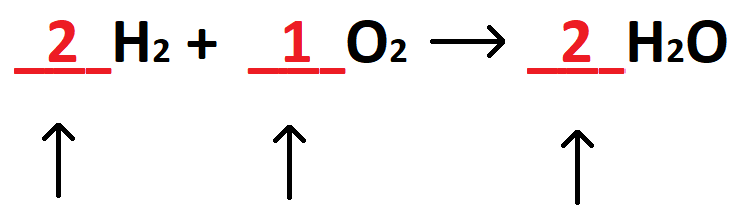
What are coefficients?
he variable that goes on the y-axis on a graph.
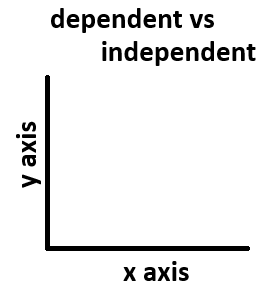
What is the dependent variable?
Jeopardy 100
Only your team may answer this question, and you get to choose how much you bet.
But be careful, if you get it wrong then you will lose that many points!
The element with 4 energy levels and 2 valence electrons.
What is calcium?
The name of CuCl2.
What is copper (II) chloride?
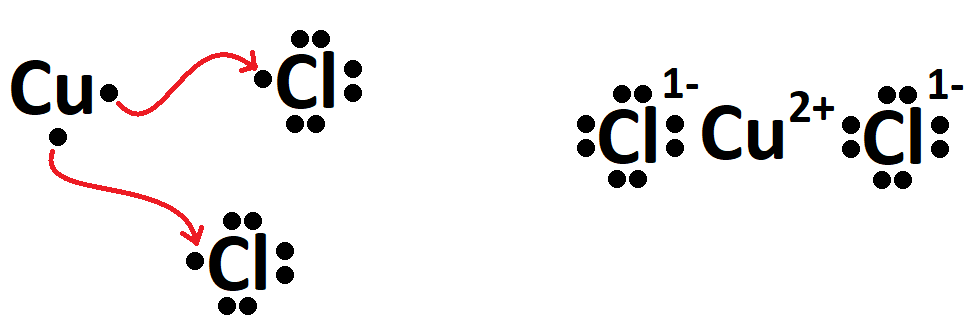
CaCO3... Mg(OH)2... NaNO3
The ionic compound that is soluble in water.
What is NaNO3?
The fourth stage of a mass spectrometer.
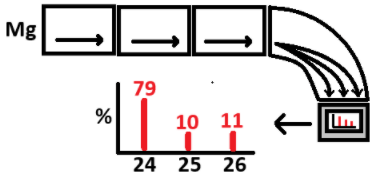
What is deflection?
The number of electrons found at X in the following 1+ cation.
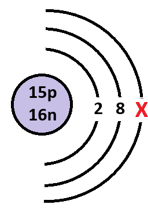
What is 4?
The number of protons, neutrons, and electrons in the following ion:
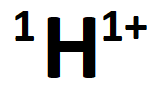
What is 1 proton, 0 neutrons, and 0 electrons?
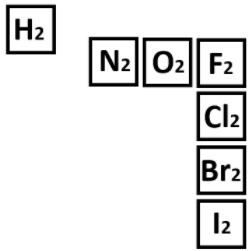
The diatomic elements.
What are H N O F Cl Br I?
The name of FeCl3.
What is iron (III) chloride?
_____N2 + _____O2 → _____NO2
The coefficients of the balanced chemical equation.
What is 1, 2, 2?
1N2 + 2O2 → 2NO2
The names of the elements used to spell this word:
What are Neon, Carbon and Potassium?
Ne C K
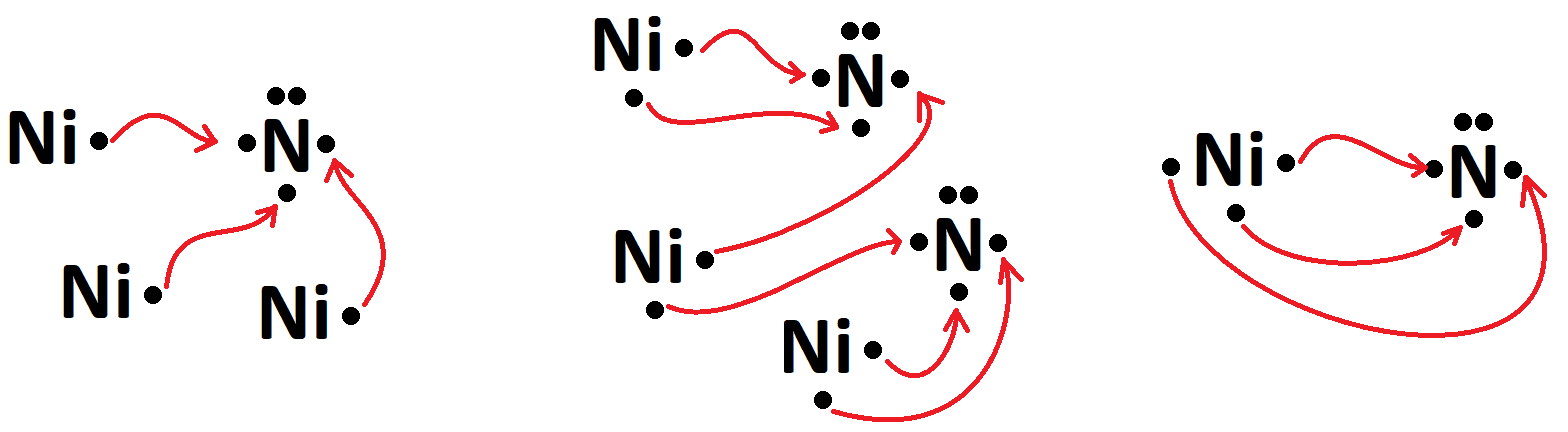
The names of Ni3N, Ni3N2, and NiN.
What are nickel (I) nitride, nickel (II) nitride, and nickel (III) nitride?
The atomic number of a 2- ion with electrons 2, 8, 8.
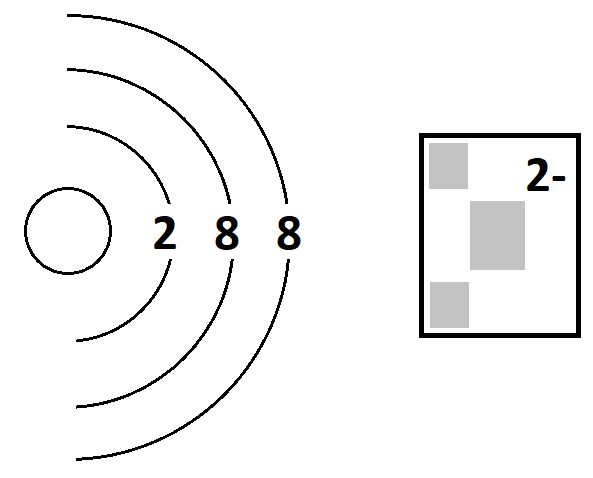
What is 16?
A = 16
The trends for atomic radius.
What is increase from top to bottom and decrease from left to right across the Periodic Table?
Jeopardy 200
Only your team may answer this question, and you get to choose how much you bet.
But be careful, if you get it wrong then you will lose that many points!
_____N2 + _____H2 → _____NH3
The coefficients of the balanced chemical equation.
What is 1, 3, 2?
1N2 + 3H2 → 2NH3
The atomic mass and charge of the ion.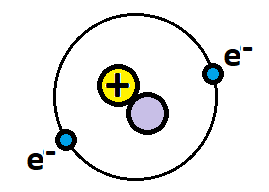
What is 2 and 1-?
A = 2
There is one extra electron, so the charge is 1-.
The number of neutrons in the following ion.
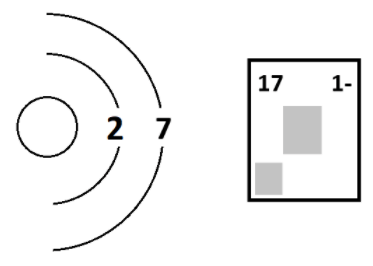
What is 9?
This ion has 1 extra electron, so the atom will have a total of 8 electrons.
An oxygen atom has 8 electrons... and 8 protons.
So it must have 17-8=9 neutrons!