Based on the BCA table, what is the limiting reactant?
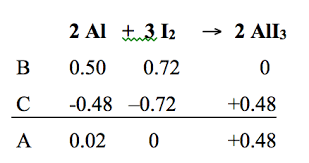
I2
Write the coeffcients for the following reaction
CH4 + O2 → CO2 + H2O
CH4 + O2 → CO2 + H2O
1 2 1 2
Name this compound
KCl
Potassium chloride
What type reaction is this?
2KI+Pb(NO3)2→2KNO3+PbI2
Double Replacement
How can we tell something is an acid from looking at a formula?
It loses protons (H+)
What element has the smallest atomic radius and what has the largest?
Helium and Francium respectively
How moles of AlCl3 will be produced? 
5 moles
Fe + H2O → Fe3O4 + H2
Fe + H2O -> Fe3O4 + H2
3 2 1 2
Name this compound
FeCl2
Iron (II) chloride
C2H5OH+O2(g)→CO2(g)+H2O
Combustion
Identify the acid
HSO4 + KOH → 2H2O+ KSO4
HSO4
Distance and magnitude of charge
Draw a particle picture to show the change in the IFE table.
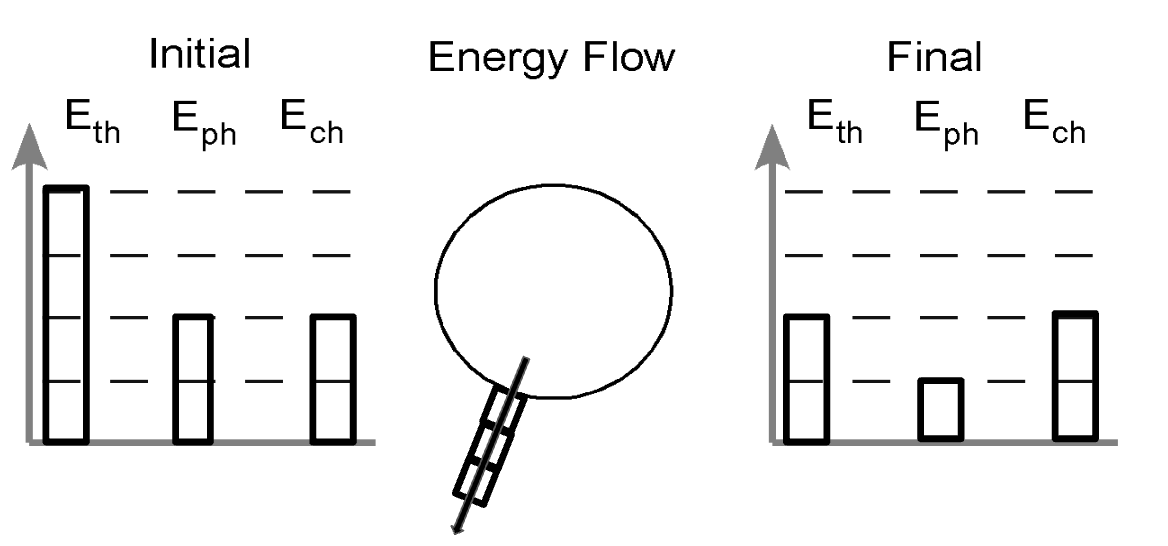
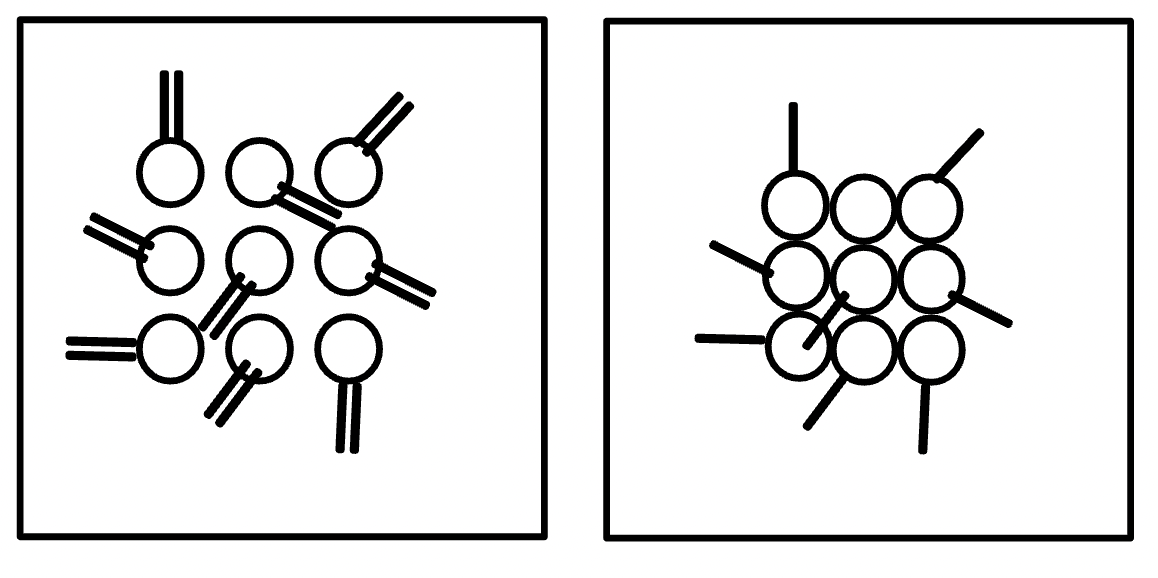
C2H6O + O2 → CO2 + H2O
C2H6O + O2 → CO2 + H2O
1 3 2 3
Name this compound
SO2
Sulfur dioxide
N2 + 3H₂ → 2NH₃
Synthesis
What is the name of this acid?
H2SO4
Sulfuric acid
Write the complete electron configuration for Krypton
1s22s22p63s23p64s23d104p6
How moles of HCL will be consumed? 
15 moles
CaCl2 + Na3PO4 → Ca3(PO4)2 + NaCl
CaCl2 + Na3PO4 → Ca3(PO4)2 + NaCl
3 2 1 6
What is the formula for the compound Lead (II) sulfate
PbSO4
2NaOH→Na2O+H2O
Decomposition
Phenophalein indicator willl turn pink in the presence of...
A base
What element has this shell model
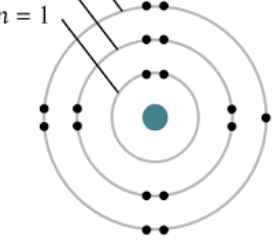
Chlorine
Calculate the grams of H2 produced 
7.5 moles (2 grams/mol) = 15 grams
NH3 + O2 → NO + H2O
NH3 + O2 → NO + H2O
4 5 4 6
What is the formula for Chromium (III) acetate
Cr(C2H3O2)3
Which type of reaction involves a pure substance reacting with a compound to form a different pure substance and a different compound
Single Replacement
When an acid an base neutralize, they will form what two products?
Water and an ionic salt
Write the abbreviate electron configuration for Po number 84
[Xe]6s24f145d106p4
Use coulomb's law and electron shells to explain the difference between Cl & Ar
Identify which has the higher electronegativity and explain why.
Identify which has the larger atomic radius and explain why.
Identify which has the higher ionization energy and explain why.
Chlorine has the higher electronegativity. It is has only 7 out of 8 valence electrons making it highly unstable as it really wants one more electron. Argon on the other hand is full so very stable.
Chlorine has the larger atomic radius. They have the same number of rings but argon has more protons. This increases the strength of the force which pulls the ring stronger making argon smaller.
Argon will have the greater ionization energy. Since it has more protons and greater force, it will be require more energy to remove its electrons.