is a well-substantiated explanation of some aspect of the natural world, based on a body of facts that have been repeatedly confirmed through observation and experiment. Such fact-supported ______ are not "guesses" but reliable accounts of the real world.
What is... a theory?
What is... Boyle's Law?
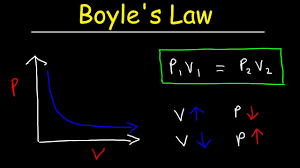 PV=PV
PV=PV
PRESSURE and VOLUME
a chemical bond that results from the sharing of valence electrons 
What is... a covalent bond?
Energy of the universe is constant (none is created or destroyed), so energy lost by a system must be gained by the surroundings and vice versa.
What is... First Law of Thermodynamics?
measure of how close a measurement comes to the actual or ture value of whatever is measured.
What is...Accuracy?
atoms move from one substance to another, but no atoms disapear or are changed in a chemical reaction.

What is... Dalton's Atomic Theory?
What is... Charles Law?
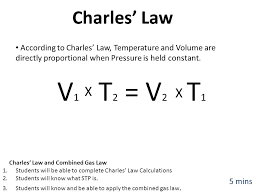 V/T=V/T
V/T=V/T
VOLUME and TEMPERATURE
a type of bond that forms when electrons are not shared equally

What is... a polar covalent bond?
Energy can be neither created nor destroyed, but it can be converted from one form to another
What is...Law of Conservation of Energy?
measurement of amount of substance.
What is... Mole?
VSEPR ~ What does it stand for?
What is...Valence Shell Electron Pair Repulsion?
What is... Gay-Lussac's Law?
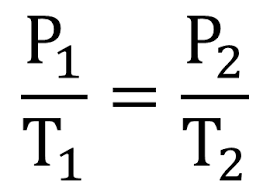 P/T=P/T
P/T=P/T
PRESSURE and TEMPERATURE
formed when a pair of electrons are shared between two atoms.
What is... a single covalent bond ?
States that rates of effusion or diffusion are inversely proportional to the square-root of a gas's molar mass.
What is...Graham's Law?
protons + neutrons
What is... Mass?
field of physics that is required to understand phenomena at the molecular and atomic levels.
 What is... Planck's Quantum Theory?
What is... Planck's Quantum Theory?
What is... Avogrado's Law?
 V/n=V/n
V/n=V/n
VOLUME and NUMBER OF MOLES
when one atom completely gives up its electrons to another atoms
What is... an ionic bond?
The pressure of a total sample of gas is equal to the sum of the pressures of each individual gas in the sample.
What is... Dalton's Law of Partial Pressures?
ions that consist of a single atom with a positive or negative charge resulting from the loss or gain of one or more valence electrons.
What is... Monatomic Ions?
discovered that when electrons lose energy, atoms should implode
What is... Niels Bohr's theory?
What is...Combined Gas Law?
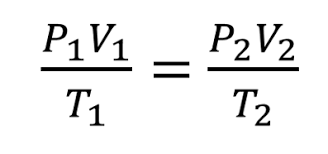 (PV)/T=(PV)/T
(PV)/T=(PV)/T
PRESSURE, VOLUME, and TEMPERATURE
all nuclei attract all of the valence e- and they are free to move in a "sea of electrons".
What is... a metallic bond?
The state of an ideal gas is determined by its pressure, volume, and temperature according to the equation
What is... Ideal Gas Law?
the study of energy changes that occur during chemical reactions and changes in state.
What is... Thermochemistry?