What is the maximum number of electrons that can fit in the 3rd shell?
18 electrons
3s - 2 electrons
3p - 6 electrons
3d - 10 electrons
the 3f subshell doesn't exist
What is the molecular shape of CH2O?
trigonal planar
Which orbital is said to be shaped like a four-leaf clover?
a d orbital
(4 out of the 5 d orbitals are shaped this way)
In the Lewis Dot Structure of SO3, how many lone pairs of electrons are on the S?
none
What is the hybridization of the N in NO3-?
sp2
Which subshell fills after the 4s subshell?
3d
What is the approximate bond angle in ozone, O3?
approximately 1200
It's actually slightly less than that because of the lone pair, but this question asked for the approximate angle.
Give a set of quantum numbers used to describe a 3p electron.
Any of these are fine:
3, 1, -1, +/-1/2
3, 1, 0, +/-1/2
3, 1, +1, +/-1/2
When you draw the Lewis Dot Structure for ClO3-, how many lone pairs of electrons are on the Cl?
1 pair
What is the hybridization of the I in ICl4-?
sp3d2
Which has the larger radius...... K or K+?
K
What is the molecular shape of ICl4-?
square planar
How many electrons can have n=3 and l=2?
10 electrons.
This is a 3d electron. There are 10 maximum electrons in a 3d subshell.
How many sigma bonds and how many pi bonds are in NO3-?
3 sigma bonds
1 pi bond
I am a molecule with a domain shape of trigonal bipyramidal and a molecular shape that is linear. What is the hybridization of my central atom?
sp3d
hint: The molecular shape is irrelevant. Do you know why?
Which has the larger radius.......K or Ca?
K
What is the molecular shape of SF4? Is it polar or nonpolar?
see-saw and polar
Which of the following forms of radiation has the highest frequency?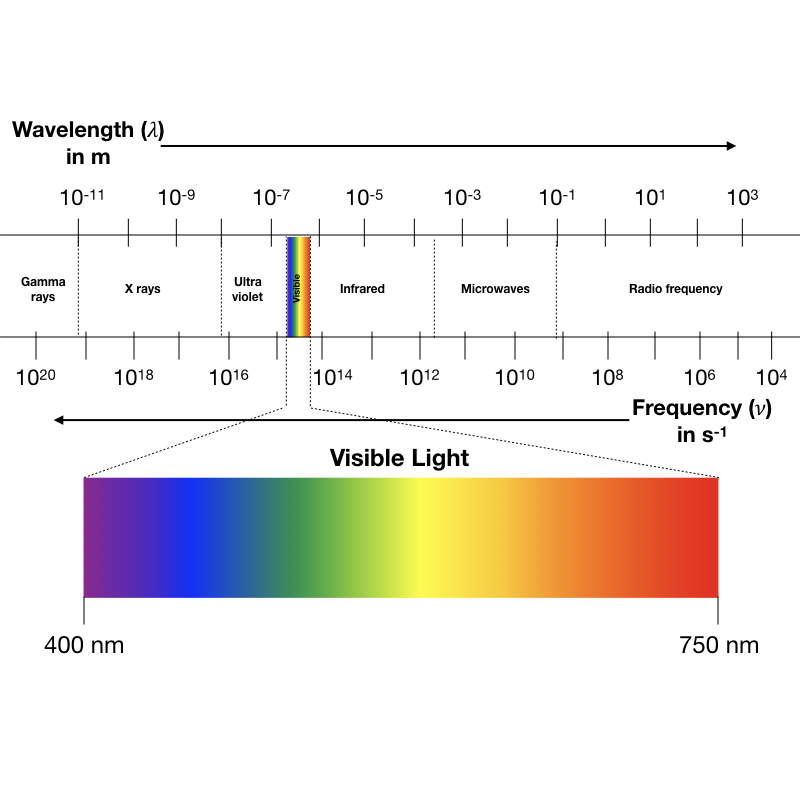
Gamma rays
(Highest frequency = shortest wavelength)
What is the average bond order of the N-O bond in NO2-?
3/2
How many unpaired electrons are in the molecular orbital diagram for O2?
2
Which has the greater first ionization energy.......Si or P?
P
What is the electron domain shape and molecular shape of BrF3? Polar or nonpolar?
domain shape: trigonal bipyramidal
molecular shape: T-shaped
Polar
What is the wavelength of blue light that has a frequency of 6.69 × 10¹⁴ s⁻¹? (c = 3.00 × 10⁸ m/s)
448 nm
Which bond is shorter......the average C-O bond in CO2 or the average C-O bond in CO32-?
The C-O bond in CO2.
CO2: both are double bonds
CO32-: one bond is double, two are single, overall average bond is 4/3
What is the bond order predicted by the molecular orbital diagram from O22-?
1