The atomic number can be found here in the periodic table
What is "above the element?"
This kind of bond is always formed between a non-metal and a non-metal.
What is a covalent bond?
Fill in the blanks:
__Fe + __O2 --> __Fe2O3
What is 4, 3, 2
When setting up our Dimensional analysis, ____ always goes on the bottom of the next fraction
What is "the units that cancel out"
This is the simplest form of a chemical formula, showing the ratio between the elements.
What is empirical formula?
This is what the atomic mass represents
What is the number of protons and neutrons
or
What is the weight of the elements
In an ionic bond, this happens to the electrons.
What is "giving away" or "taking"
This kind of reaction always has oxygen as a reactant and releases heat and energy.
What is combustion?
Using Dimensional Analysis, this many dozens is in 48 cookies.
What is 4 dozens?
the electron configuration of Titanium
What is [Ar]4s23d2
This is how much an element wants electrons.
What is electronegativity?
A lewis dot structure of Sulfur and how many electrons it needs to be complete
What is (drawing) and 2 electrons
This kind of reaction is:
Ca(NO3) + KBr --> CaBr2 + KNO3
What is double replacement?
This many atoms are in 3.81 moles of Na.
What is 2.29 x 1024 atoms
The percent composition of H2O.
This is the trend for electronegativity.
What is "increasing to the right, decreasing going down"
Ca2+ and NO2- forms what compound?
What is Ca(NO2)2
The product(s) of this single replacement reaction:
KCl + Mg --> ?
What is MgCl2 and K
This many moles is in 5 grams of NaOH.
what is 0.125 moles?
The molecular formula for an element with molar mass of 78.1g and an empirical formula of CH
What is C6H6
The average atomic mass of this: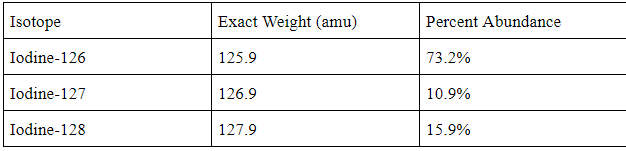
What is 126.3 amu?
A drawing of what happens when Magnesium and Fluorine tries to bond.
What is "a drawing??"
The balanced equation for the combustion of C3H8
What is
C3H8 + 5O2 --> 3CO2 + 4H2O
This many atoms are in 0.32 grams of Aluminum.
What is 7.14 x 1021 atoms?
The Empirical formula for a compound with 52% Zinc, 9.6% Carbon, and 38.4% Oxygen
What is ZnCO3?