This subatomic particle is found in the nucleus and has a positive charge
Protons
How many protons are in the carbon atom?

6
Bonus question for another 100 points: What is the name for that number?
CO2
Compound
Salt dissolved in water
Mixture
Sand mixed with water
Heterogeneous
This subatomic particle is not found in the nucleus and has a negative charge
Electrons
What is the atomic mass of Fluorine?

18.998 (will also accept rounded answers)
O3
Element
Glucose (C6H12O6)
Pure Substance
Shampoo

Homogeneous
This subatomic particle is found in the nucleus and has a neutral charge
Neutrons
Approximately how many neutrons are present in a Neon atom?

10
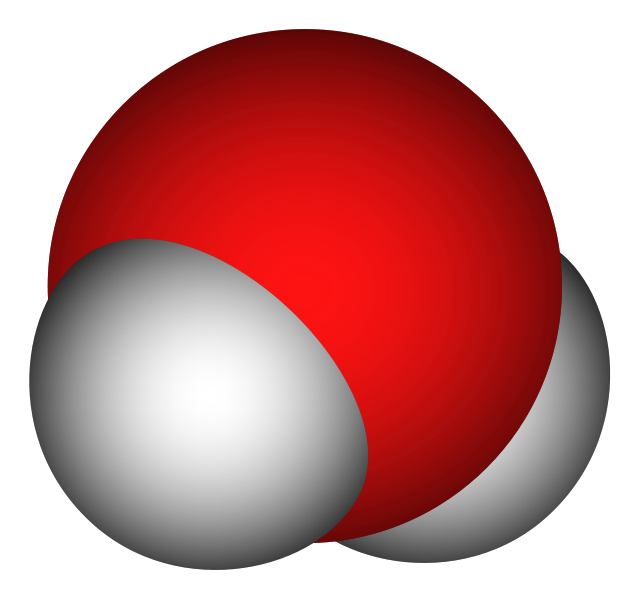
Compound
A mixture is something that can be separated using ____________ means
Physical
Which of the following is a homogeneous mixture?

A
This subatomic particle is responsible for determining what element the atom is.
How many electrons does an iodine atom need in order to be a neutral atom?

53
Which of these is does not contain elements

C
Which of the following contains pure substances only?

B and D
A fizzy soda
Heterogeneous
This subatomic particle is responsible for reactivity with other atoms.
Electrons
Which pair of elements listed below have properties that are most similar to each other?
1. Boron (B) and Carbon (C) 2. Argon (Ar) and Xenon (Xe) 3. Fluorine (F) and Chlorine (Cl) 4. Copper (Cu) and Tin (Sn)

3. Fluorine (F) and Chlorine (Cl)
Which of these contain only elements?
B and E
Which of the following are mixtures?
B, C, D, E, F, H
What is another name for a homogeneous mixture?
A solution

