A form of a chemical element that has the same number of protons but a different number of neutrons compared to other forms of the same element
What is an isotope?
22.4 L.
What is the volume that one mole of an ideal gas occupies at STP?
Bonds formed between positively charged cations and negatively charged anions.
What are ionic bonds?
NaCl
What is the name chemical formula for sodium chloride or table salt?
A reaction where there are two reactants that form one compound as a product.
What is a synthesis reaction?
The electrons in the outermost shell of an atom.
What are valence electrons?
Avogadro's Number.
What is 6.02x1023?
Bonds formed by overlapping orbitals, or "sharing electrons"
What is covalent bonding?
Diphosphorus pentoxide.
The reaction of Na3PO4 + 3 KOH 🡪 3 NaOH + K3PO4.
What is double replacement?
Columns on the periodic table that have the same number of valence electrons.
What are groups?
The molarity of 0.060 moles NaHCO3 in 1.500 L of solution.
What is 0.04 M?
How metals form solids and conduct electricity.
What is a sea of electrons?
Iron (III) chloride
What is the name for FeCl3?
The solubility of Ca(NO2)2.
What is insoluble?
An atom's ability to attract shared electrons in a chemical bond.
What is electronegativity?
The number of moles of NaCl contained in 0.500L of a 1.5M solution.
What is 0.75 mol NaCl?
The type of bond in NO2.
What is a covalent bond?
CuO.
What is copper (II) oxide?
The balanced precipitation reaction of CaCl2 and K3PO4.
What is 3 CaCl2 (aq) + 2 K3PO4 (aq) → 6 KCl (aq) + Ca3(PO4)2 (s) ?
The Lewis Structure of CH3OH.
What is 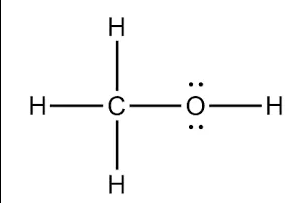
40.0% carbon, 6.7% hydrogen, and 53.5% oxygen.
What is the percent compositions of CH₂O?
Ionic Solids are formed this way.
What is formed by all cations being bonded to all anions?
Ammonium sulfate.
What is (NH4)2SO4?
The NET ionic equation of CaCl2 and K3PO4.
3 Ca2+ (aq) + 2 PO4 3- (aq) → Ca3(PO4)2 (s)