carbon monoxide
CO
What is HCN electron & molecular geometry
linear
Na2O
P2O
FeO
CaC2O4
XeF5
Na2O -- Sodium Oxide
P2O -- diphosphorus monoxide
FeO -- iron(II) oxide
CaC2O4 -- calcium oxalate
XeF5 -- xenon pentafluoride
BH3
trigonal planar, nonpolar
Which substance would you expect to have the highest boiling temperature? Why?
a) CCl4
b) CHCl3
b) CHCl3
Trichloromethane is polar. The molecules attract each other so more energy (higher temperatures) is needed to boil the liquid.
lead(IV) hydroxide
Pb(OH)4
Which molecule would have the shape shown below?
a) H2O b) CO2 C) NH3 D) CBr4
Answer: NH3

SO2
angular, nonpolar

Which of the molecules are polar?


 CBr4
CBr4
H2O --  NH3 --
NH3 -- 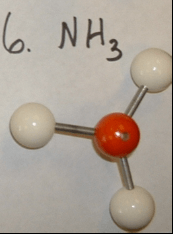
sodium oxalate
Na2C2O4
What shape is the following structure?
tetrahedral or trigonal pyramidal.
trigonal pyramidal
CF4
tetrahedral,nonpolar
CH3Cl
Polar
Sulfur Hexafluoride
SF6
Sort Molecules by Shape
PCl3, NH3, BI3, SO3, O2, SiS2, CO2, BeH2
NH3, PCl3, SO3, HCO2-, BI3, SF2, CF4, SiH4
CF4
SiH4
Linear -- BeH2, O2, SiS2,CO2
Trigonal pyramidal -- PCl3, NH3
Trigonal planar -- BI3, SO3, HCO2-
Angular (bent) -- SF2
Tetrahedral -- CF4, SiH4
CH2O
trigonal planar, polar
What are metaliods?

Si
B
Ge
Po
Potassium Permanganate
KMnO4
Which molecule would have the shape shown below?
a) H2O b) CO2 C) NH3 D) CBr4

CO2 -- Carbon Dioxide
CH3OCH3
polar
CH3Cl
tetrahedral, polar

What are metals?

Zn, Hg, Ag. Fe, Al, Pb, Po, Ni, Li, Bi, Ba, Rb, Be