Chemistry and Nature of Science
Scientific Notation
Accuracy, Precision, and Certainty
Measure It
Unit Conversions and Derived Units
100
Chemistry is the study of...
matter and how it changes
100
The shortcut method for writing very small numbers or very large numbers.
Scientific Method
100
Difference between accuracy and precision
Accuracy - how close a measurement is to the actual answer
Precision - how close a measurement is to other measurements of the same thing
100
This is the measurement according to the following ruler. 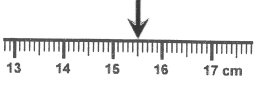
What is 15.5 cm?
100
Kilo- means...
1,000 times
200
Matter is anything that has....
mass and volume
200
Example of Scientific notation and Standard notation
229,000 (Standard)
4.73 x 10`3 (Scientific)
200
What number of this measurement is uncertain?
124.8
8
200
This is the measurement according to the following ruler. 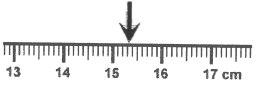
What is 15.3 cm?
200
500 mg = _____ g
0.5 grams
300
Difference between Qualitative and Quanitative answers
Qualitative - Describing something with out using numbers.
Quantitative - Describing something using numbers.
300
245,860,000,000
Place in Scientific Notation
2.4586 x 10`11
300
How many significant figures are in the measurement 30,780 grams?
4
300
This is the temperature according to the following thermometer.
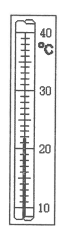
What is 22 degrees Celsius?
300
10 km = ____ m
10,000 m
400
The method of study used by scientists
Scientific Method
400
2.65 x 10`-12
Standard Notation
.00000000000265
400
How many significant figures are in the measurement 3050.1 grams?
5
400
This is the measurement on the following graduated cylinder. 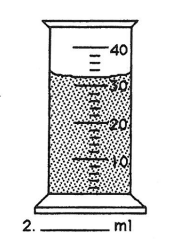
What is 32 ml?
400
150 g = _____ kg
.150 kg
500
Difference between independent and dependent variable
Dependent - responds to the independent variable Independent - changed by the experimenter
500
What does a negative exponent tell you about a number?
The number is very small.
500
A three is 70 feet tall. It was measured twice using the same tool. The measurements recorded were: 59.3 feet and 58.9 feet.
Are these measurements accurate, precise, or both?
Precise
500
This is the following graduated cylinder drawn with 7.6 ml in it. 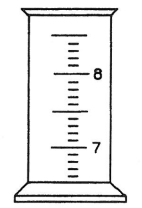
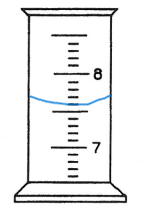
500
2.5 km = ______ cm
250,000 cm