When balancing chemical equations you need to have the same of these two things on either side of the reaction.
What are reactants and products?
Where is John Dalton from?
What is the UK or Great Britain?
This is the molecular structure of water.
What is H2O?
Who wrote blank space and in which year?
Who is Taylor Swift and 2014?
The particles in a chemical reaction are either of these two types.
What are molecules or atoms?
Two part question: John Dalton made an original model of water, it had this many atoms and these types of atoms.
What was two, one hydrogen and one oxygen?
This property of matter suggests how much you can bend a metal.
What is malleability?
This is one of the molecules that results from combining citric acid, sodium bicarbonate and water.
What is CO2?
Weathering is this type of change.
What is physical?
When balancing chemical equations you can change only this factor
What is the coefficient?
John Dalton was responsible for identifying this idea that molecules could be rearranged.
What is a chemical reaction?
This property of matter explains the ability of an object to sink or float.
What is density?
The three atoms that make up most of the molecules available on earth are these.
What are carbon, hydrogen and oxygen?
Dissolving is this type of process.
What is a physical change?
You always need more than one reactant for a chemical reaction to take place. Give an example.
What is false? Because water can be decomposed into hydrogen and oxygen?
This is the idea that matter can neither be created nor destroyed.
What is the law of conservation of matter/mass?
This physical aspect can be changed for gases but not for liquids or solids.
What is volume?
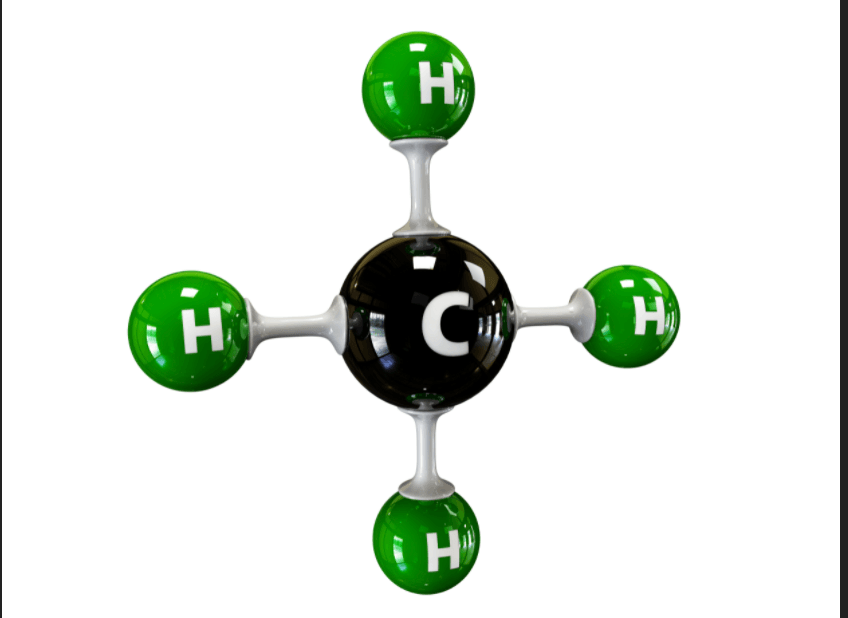 Methane, a greenhouse gas would be called by this chemical name and is produced in abundance by this animal.
Methane, a greenhouse gas would be called by this chemical name and is produced in abundance by this animal.
What is CH4 and cows? OR What is one carbon bonded to four hydrogen?
Chemical changes produce this as their products.
What are new substances (or different arrangements of the same molecules) with different properties?
The balanced chemical equation for this.
KClO3 → KClO4 + KCl
What is 4KClO3 → 3KClO4 + KCl?
What is they both contain the elements hydrogen, oxygen and carbon?
Two Part -> These two properties enabled us to identify the difference between helium, air, and carbon dioxide (bath bomb gas).
What are density and flammability?
CO2 is considered this type of gas when it is found in the atmosphere.
What is a greenhouse gas?
Electrolysis produces these two molecules and in this ratio.
What is molecular hydrogen and molecular oxygen in a 2:1 ratio?
The balanced chemical equation for this.
SiCl4 + H2O → H4SiO4 + HCl
What is SiCl4 + 4H2O → H4SiO4 + 4HCl ?
This was the result of combining hydrogen gas and oxygen gas and adding a spark.
What is an explosion with the product being water?
This is true about the products of a chemical reaction when compared to the reactants.
What is they have different properties from one another?
Molecules can either be made of the _______ atoms or _______ atoms.
What is the same types of atoms or different types of atoms?
We can identify the difference between hydrogen and oxygen by completing a flammability test and observe these two different results.
What are hydrogen exploding and oxygen igniting an ember?