The first column or group of elements which includes Lithium, Sodium and Potassium is?
Alkali Metals
Isotopes of the same element have the same atomic number but a different atomic ________ ________.
mass number or mass units
The three sub-atomic particles are?
Protons, neutrons and electrons
What element is depicted below?
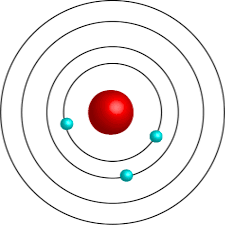
Lithium (Li)
What is the number "3" in this compound called?
3Fe(OH)2
The coefficient
How many valence electrons do each of the elements in the Alkaline Earth Metals family have?
2 valence electrons
Some isotopes are stable while others are __________.
Radioactive
What two sub-atomic particles can be found in the nucleus?
Protons and Neutrons
What element is depicted below?

Carbon (C)
What is the number "2" in this compound called?
3Fe(OH)2
The subscript
The Halogen family (column #17) represent three states of matter - solids, liquids and gasses. Which halogen is a liquid at room temperature?
Bromine (Br)
What element (and its isotopes) is depicted below?
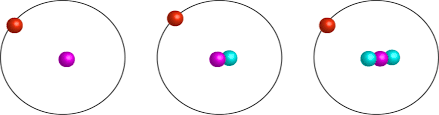
Hydrogen
(The isotopes are Protium, Deuterium and Tritium)
Where can electrons be found in an atom?
On the rings, shells or orbits.
Which energy level is closest to the nucleus?
n = 0, n=1, n=2, or n=3
n = 1; there is no energy level known as n = 0
How many total atoms are present in this compound?
5Fe3O4
15 Iron (Fe) atoms + 20 Oxygen (O) atoms = 35 total atoms
These metals located in the center of the periodic table are what people commonly associate as "metals" due to their widespread use.
Transition metals
The hyphens used in isotopes, like Magnesium-24, Magnesium-25, and Magnesium-26 indicate the _______ ________ present for each isotope.
atomic mass
Which sub-atomic particles enables atoms to bond with other atoms?
Electrons or valence electrons
The energy needed to remove an electron from an atom is called the _________ _________. 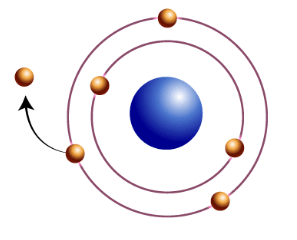
Ionization Energy
How many total atoms are present in this compound?
2Al2(CO3)3
4 Aluminum (Al) atoms + 6 Carbon (C) atoms + 18 Oxygen (O) atoms = 28 total atoms
"Krypton," "Xenon," and "Radon" are not far away stars; rather they are members of this family of elements.
Noble gasses
Which isotope of Oxygen is the most commonly found oxygen on Planet Earth?
Oxygen-16
The atomic number for a neutral element equals the # of ________ or ________.
protons and electrons
The tendency of an atom to want to gain electrons is known as its _______________.
Electronegativity
How many total atoms are present in this compound?
4Sn(NO3)2
4 Tin (Sn) atoms + 8 Nitrogen (N) atoms + 24 Oxygen (O) atoms = 36 atoms