What is chemistry?
the scientific study of the composition, structure, properties, and changes of matter
What are the 3 subatomic particles that make up an atom?
Protons, neutrons and electrons
What does it mean for an atom to be neutral, and what is required for this condition?
A neutral atom is an atom that has an equal number of protons and electrons, resulting in no overall charge.
Which subatomic particles are directly influenced by the electromagnetic force and why?
Protons and electrons because the electromagnetic force only acts on charged particles.
What is matter? Provide an example.
Matter is anything that has mass and volume.
This means it can be weighted and it occupies space.
Examples may vary.
Compare the charge and location of protons, neutrons, and electrons within an atom.
Protons = positive, nucleus
Neutrons = no charge, nucleus
Electrons = negative, electron cloud
Define an isotope and provide an example for Nitrogen.
An isotope is an atom with the same number of protons but a different number of neutrons, resulting in a different mass number.
Example: Nitrogen with a mass that is not 14, like Nitrogen-15
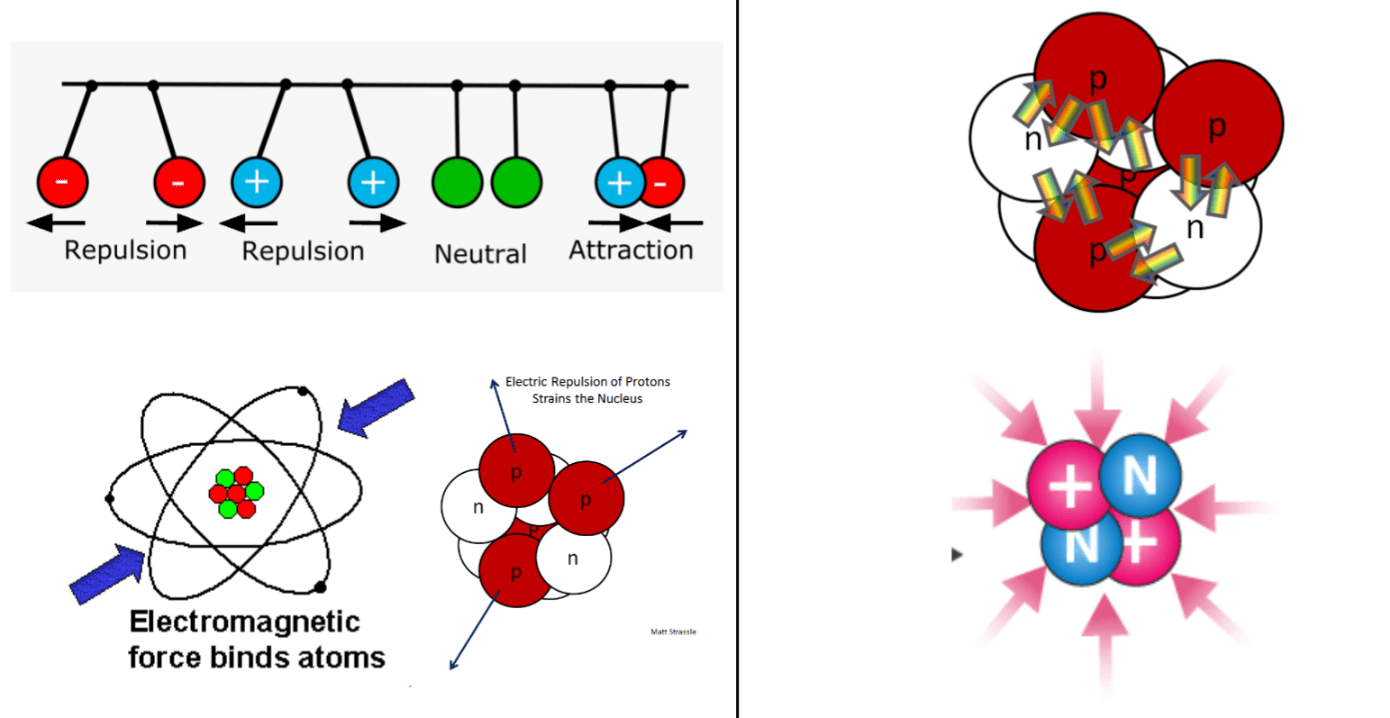
What's the role of the electromagnetic force and the strong nuclear force?
Electromagnetic force = bind the atom / hold protons and electrons together within it
Strong nuclear force = bind the nucleus / prevent the protons from breaking the nucleus apart
Name the three categories of nonmatter. Provide an example of each.
If something is not matter, it can be a force, energy, or abstract concepts.
Examples may vary.
Compare the role of the 3 subatomic particles on the atoms' weight and volume.
Weight = protons and neutrons as they each weigh 1 amu and electrons weigh 0 amu
Volume = most of the atom consists of empty space or the space where electrons orbit
What is an ion, and how is it formed?
An ion an atom that has gained or lost one or more electrons, resulting in a net positive or negative charge.
Create a model (or 2) demonstrating the directions in which the electromagnetic and strong nuclear forces work.
Sort the following items into matter and nonmatter: smoke, rainbow, lead, and happiness.
For the nonmatter, determine what category it falls into.
Matter = smoke, lead
Nonmatter = happiness (abstract concept), rainbow (energy)
Define the average atomic mass, mass number, and atomic number. If relevant, explain how you get each.
Average atomic mass = the mass found at the bottom of the chemical symbol box that considers all versions of the same element
Mass # = protons + neutrons (or round the AAM)
Atomic # = # of protons
Write the isotopic notation for carbon-14 and explain how to interpret it.
 14 = mass (p's and n's)
14 = mass (p's and n's)
6 = element (p's)
Compare and contrast the relative strength and range of the electromagnetic and strong nuclear forces.
The strong nuclear force is ~130 times stronger than the electromagnetic force.
The electromagnetic force has a longer range than the strong nuclear force, the latter of which only works at extremely small distances.
Create a model that breaks down matter into 2 smaller components and defines them.
Matter can be broken down into:
- elements (pure substances consisting of only one type of atom)
- atoms (the smallest unit of an element that retains its physical and chemical properties)
An element has an atomic number 6, mass number 13, and a charge of +2.
Calculate the number of protons, neutrons, and electrons.
Protons = 6 (atomic #)
Neutrons = 13 - 6 = 7 (mass # - atomic #)
Electrons = 4 (do the opposite of the charge to the protons)
Atom #1 has 5 protons, 6 neutrons and 6 electrons.
Atom #2 has 6 protons, 7 neutrons and 6 electrons.
Are they isotopes of each other? Explain your reasoning.
No, they are not isotopes of each other because they are not the same element (different # of p's). To be isotopes they need to:
- be the same element
- have different masses (# of n's)
How does the number of neutrons affect stability and why?
Too many neutrons = unstable, nucleus too big, weakened strong nuclear force
~ a good proton-to-neutron ratio (usually a 1:1) = stable atom
Too few neutrons = unstable, too much proton repulsion given lack of buffers