What is the SI unit of pressure?
Pascal
This law says that the volume of a gas is directly proportional to its absolute temperature at constant pressure.
Charles’s Law
What is the maximum number of electrons that occupy f sublevel?
7 orbital * 2 electrons=14
What type of bond is formed when atoms share electrons?
Covalent bond
What are isomers?
Compounds with the same formula but different structures.
Which instrument is used to measure air pressure?
True/False
According to kinetic theory, pressure results from this action of gas molecules on container walls.
True
Pressure in gases, according to KMT, comes from the continuous and elastic collisions of moving gas particles with the container walls.
Two electrons occupy the same 4p orbital. Using quantum numbers, explain how their spins differ.
Their ms values must be opposite, one + 1/2and one −1/2, according to the Pauli Exclusion Principle.
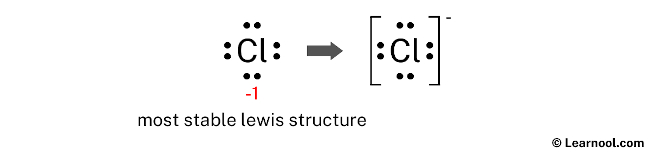
Draw the Lewis structure of the chloride ion (Cl⁻) and explain why it carries a negative charge.
Classify the type of isomerism (if any) shown by the compounds below.
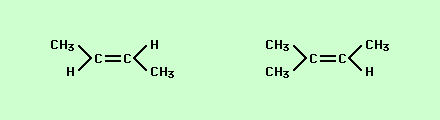
Not an isomer.
A force of 120 newtons (N) is exerted uniformly on an area of 0.6 square meters (m²).
What is the pressure of the air acting on the surface in pascals (Pa)?
200 Pa
In PV = nRT, this letter stands for the gas constant, typically 0.082057 L·atm·mol⁻¹·K⁻¹.
It is the R or the ideal gas constant.
An electron has n=4, l=1, ml=0, ms= +1/2. Predict how many other electrons can share the same n and l but differ in ml and ms.
There are 5 other electrons:
l=1 (p-orbital) has 3 possible ml values (-1, 0, +1). Each ml can hold 2 electrons with opposite ms.
Draw the Lewis structure of CO₂. How many double bonds are in the molecule?
2 double bonds
What is positional isomerism?
Isomers differ in the position of a functional group or double bond or substituent.
Why does pressure increase when the area decreases?
Because the same force acts on a smaller area.
A 1.00 L flask of gas at 2.00 atm is heated from 300 K to 450 K at constant volume. What is the final pressure
P₂ = P₁ × T₂/T₁
= 2.00 × 450/300 = 3.00 atm
If an electron has (n=5, l=2, mₗ=0, mₛ=+½), what orbital is it in?
5d orbital
Which atom is usually placed at the center of a Lewis structure?
The least electronegative atom.
Classify the type of isomerism (if any) shown by the compounds below.
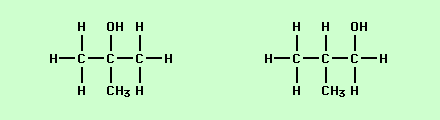
Positional Isomerism
A diver’s air bubble is 3.0 L at 1 atm. What is its volume at 3 atm? (Use P₁V₁=P₂V₂)
1.0 L
A 5.00 L cylinder contains nitrogen at 2.00 atm and 273 K. How many moles are present?
n = PV/RT
= (2.00)(5.00)/(0.08206×273) ≈ 0.446 mol
Given an electron in a 3d orbital, justify why it cannot have l=3.
For a given n, ℓ must satisfy 0 ≤ ℓ ≤ n−1. For n=3, ℓ can only be 0, 1, or 2; ℓ =3.
When an atom cannot achieve an octet of electrons, it does not become stable. What usually happens to it?
It becomes reactive.
Are the compounds shown below isomers?
Yes