1. Flammability is an example of a _________________
a. physical property
b. chemical property
1. La inflamabilidad es un ejemplo de _________________
a. propiedad fisica
b. Propiedad quimica
What is chemical property
6. Density
What is physical, intensive property?
11. Water boiling
What is a physical change?
16. Cu
What is copper?
21.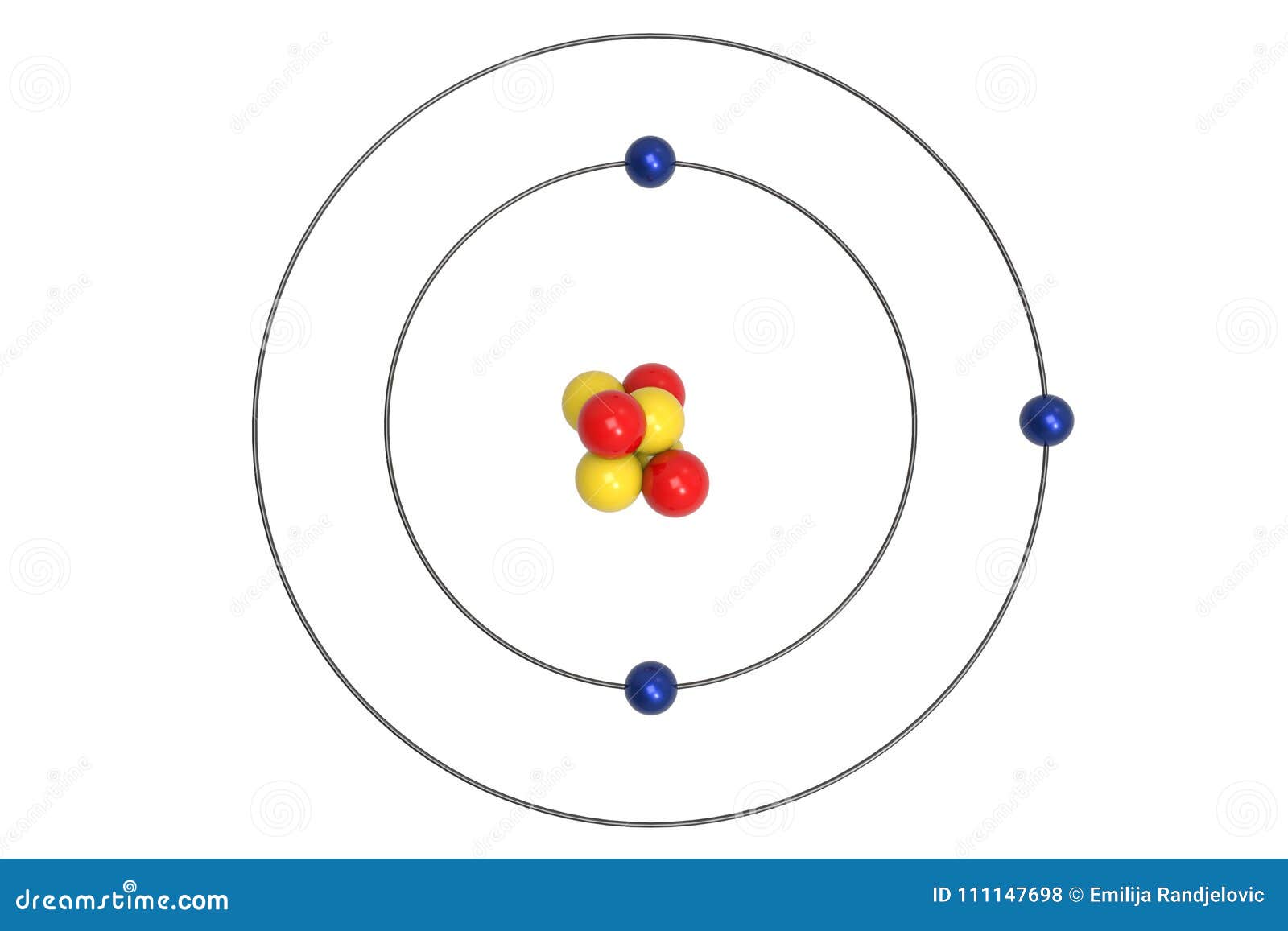
lithium-6
2. Freezing point is an example of a ________________.
a. physical property
b. chemical property
What is physical property.
12. A nail rusting
What is a chemical change?
22.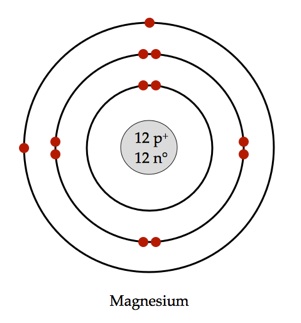
Magnesium-24
3. Formula and units for density.
What is mass/volume and g/cm3
13. Wax melting
What is a physical change?
23.
potassium-39
4. A lead cube has a density of 10g/cm3, you cut it in half, what is the density now?
10g/cm3
14. What is a good indicator for a chemical change?
What is change of color, or production of heat or light.
24.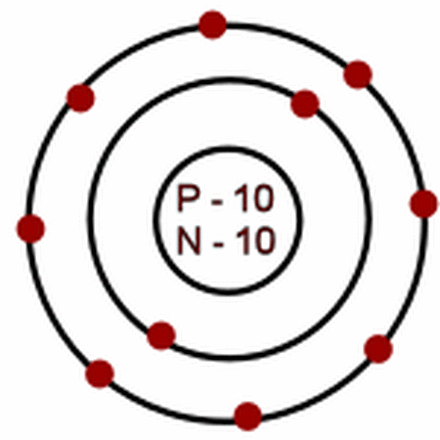
neon-20
5. Where are protons, neutrons, and electrons found and how does each subatomic particle effect an atom?
protons and neutrons are in the nucleus of an atom and the electrons are found in clouds outside of the nucleus called quanta.
Protons and neutrons determine the mass and electrons and protons determine its charge.
10. Mass
What is physical, extensive property?
15. What is a good indicator for a physical change?
What is change of state?
25.
lanthanum-139