What are the elements in Group 18 called ?
Noble Gases
The simplest form of matter is the
Atom
Who was John Dalton & what did he do ?
English chemist who formulated an atomic theory and the law said that all atoms of an element are identical, chemical reactions occur when atoms recombine in new ways, simple whole numbers
Define the following terms:
Fission
Fussion
fission - Splitting of a nucleus
Fusion - joining of 2 nuclei
What is an Ionic Bond?
A bond where electrons are transferred between a metal and a nonmetal.
What is atomic mass ?
total weight of protons and neutrons
What does this diagram represent
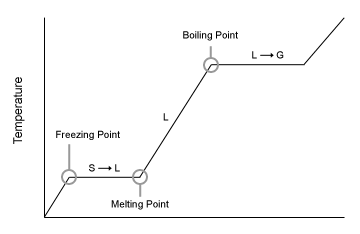
A heating Curve
What did J.J Thompson do ?
used the cathode ray tube to discover electrons
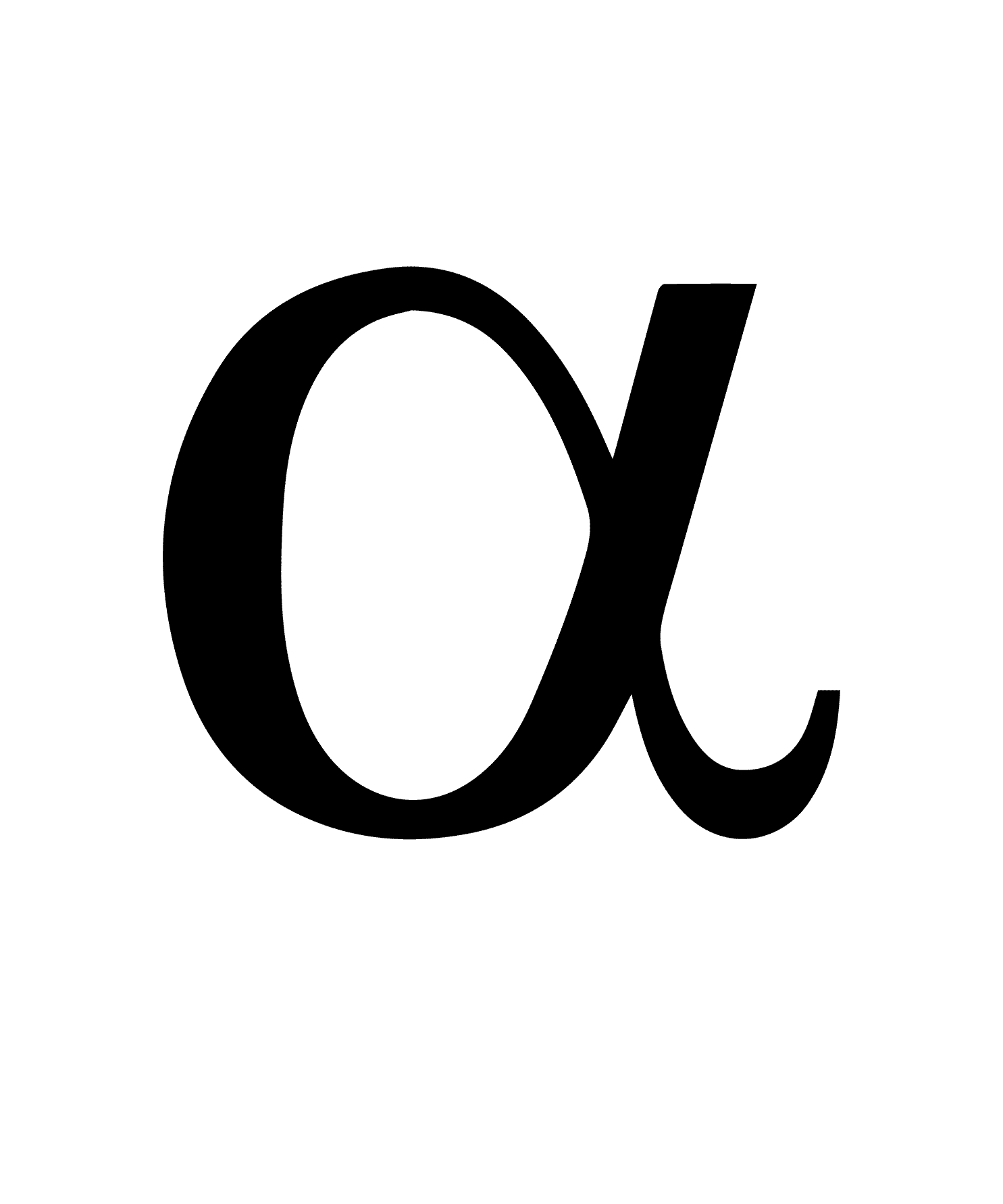 what does this symbol represent
what does this symbol represent
Alpha particle or Alpha decay
A diagram representing the Valence electrons of an element or compound
Lewis dot structure
What is Bohr's Diagram ?
a diagram of an atom which includes all protons, neutrons, electron shells, and electrons
When a substance is changing from a solid to a liquid, what type of energy exists?
What is this transition called?
Potential energy exists when a substance is changing from a S- L.
This transition is called a phase change.
He was known for his gold foil experiment- fired negative ions at thin sheet of gold foil, discovered the atomic nucleus and proposed a nuclear model of the atom , who is he ?
Ernest Rutherford
When a beta particle and positron combine, what particle is made? −1𝑒 0 + +1𝑒 0 → ?
A) proton. B) neutron.
C) gamma ray. D) beta particle.
E) alpha particle.
C
What type of bond is produced by sharing electrons?
Covalent bonds
What is an element ?
A molecule composed of one kind of atom; cannot be broken into simpler units by chemical reactions.
Give an example of an element and a compound
Is Water Heterogeneous or Homogeneous?
Are Fruit loops Heterogeneous or Homogeneous?
Element = Carbon.....
Compound = H2O
Water = homogeneous
Fruit loops = Hetereogenous
Thompson's model was called ?
The Plum Pudding Model
33. Radium-226 decays by alpha decay to
A) barium-131. B) cobalt-60.
C) carbon-14. D) polonium-218.
E) radon-222.
E
What is electronegativity? What type elements will have low electronegativity? WHat type of elements will have a high electronegativity?
The desire to gain electrons. Metals have low electronegativity and non metals have High electronegativity.
What are the horizontal rows on the periodic table called ?
Periods
What are the 3 subatomic particles and where are they located?
Neutrons - inside the nucleus
Electrons - outside the nucleus
Protons - Inside the nucleus
Who was Democritus ?
Greek philosopher that said all matter is made of tiny, indestructible particles called atoms
46. Sodium-24 has a half-life of 15 hours. How many hours is three half-lives?
A) 60 hours B) 45 hours
C) 30 hours D) 15 hours
E) 7.5 hour
B
What are 2 ways to identify a bond as ionic or covalent?
1. Determine types of elements that are bonding
m + nm = ionic
nm +nm = covalent
Electronegativity Difference greater than 1.7 = ionic
less than 1.7 = covalent